
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
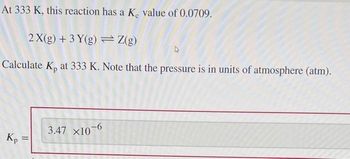
Transcribed Image Text:At 333 K, this reaction has a Ke value of 0.0709.
2 X(g) + 3 Y(g)
Z(g)
Calculate K₂ at 333 K. Note that the pressure is in units of atmosphere (atm).
Kp
=
3.47 X10-6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the following reaction: A(g) + B(g) = C(g) Calculate the equilibrium constant K given the following information: The initial pressure of A is 4.421 bar and the initial pressure of B is 3.829 bar The equilibrium pressure of C is 1.699 bar.arrow_forwardChoose the letter of the correct answer. N204 (g) = (reversible to) 2NO2 (g), K = 4.63 x 10-3 I. the given is equation is an example of homogenous equilibrium II. Equilibrium will lie to the right Which statement(s) is/are correct?arrow_forwardConsider this reaction in a closed container. N₂O,(g) 2NO₂(g) = An equilibrium mixture is prepared at 298 K with these partial pressures: N₂O, = 3.476 atm, NO₂ 1.048 atm. Suddenly, just the NO₂ partial pressure is decreased by a factor of 2.6 In which direction do you predict the reaction will go to return to equilibrium? Calculate AG for this non-equilibrium mixture before any further reaction occurs. D Plus or minus 5% J/mol 3 Attempts Submitarrow_forward
- At 429 K, this reaction has a K. value of 0.0407. X(g) + 2 Y(g) = Z(g) Calculate Kp at 429 K. Note that the pressure is in units of atmosphere (atm). Kp =arrow_forwardConsider the following chemical equilibrium: C(s) + 2 H₂ (g) ⇒ CH₂(g) Now write an equation below that shows how to calculate K from K for this reaction at an absolute temperature T. You can assume I is comfortably above room temperature. If you include any common physical constants in your equation be sure you use their standard symbols, found in the ALEKS Calculator. K -0 X 00arrow_forwardThe reaction C(s) + 2 H₂(g) = CH₂(g) has Kp = 0.263 at 1000. K. Calculate the total pressure at equilibrium when 4.305 g of H₂ and 22.06 g of C(s) are placed in a 9.08 L flask and heated to 1000. K. Ptotal = Calculate the total pressure when 4.305 g of H₂ and 7.404 g of C(s) are placed in a 9.08 L flask and heated to 1000. K. Ptotal = atm atmarrow_forward
- Consider the equilibrium system described by the chemical reaction below. If the partial pressures at equilibrium of NO, Cl2, and NOCI are 0.095 atm, 0.171 atm, and 0.28 atm, respectively, in a reaction vessel of 7.00 L at 500 K, what is the value of Kp for this reaction? 2 NO(g) + Cl2(g) = 2 NOCI(g)arrow_forwardAn experimenter places the following concentrations of gases in a closed container: NOBI]=0.245 M, [NO] = 3.29 x 10 M, Br2] = 2.02 x 10- M These %3D gases then react: 2NOB1(g) 2NO(g) + Br2 (g) 4. At the temperature of the reaction, the equilibrium constant K, is 5.85 x 10 * Calculate the reaction quotient, Q, from the initial concentrations and determine whether the concentration of NOBR increases or decreases as the reaction approaches equilibrium. Q.= %D The concentration of NOBRarrow_forwardConsider the reaction: 2 H2S (g) ⇋ 2 H2 (g) + S2 (g) Kp = 2.4 x 10 -4 at 1073 K If the initial pressure of hydrogen sulfide is 755 torr, what will be the equilibrium partial pressure of each gas?arrow_forward
- At 487 K, this reaction has a Ke value of 0.0463. 2 X(g) + 3 Y(g) = 2Z(g) Calculate Kp at 487 K. Note that the pressure is in units of atmosphere (atm). Kp =arrow_forwardFor the reaction 2 A(g) = B(g) + 3 C(g), we begin with only pure A(g) and the total pressure is 8.00 atm. We attain equilibrium. At equilibrium, the total pressure is 12.00 atm. What is the value of ∆G◦ for this reaction? The temperature is 25.0 ◦C throughout.arrow_forwardConsider the following reaction where K, = 6.50x10-3 at 298 K. 2NOBR(g) =2NO(g) + Br2(g) A reaction mixture was found to contain 8.79x10-2 moles of NOBR(g), 2.03x10-2 moles of NO(g), and 4.53x10 2 moles of Bro(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qc, equals The reaction A. must run in the forward direction to reach equilibrium. B. must run in the reverse direction to reach equilibrium. C. is at equilibrium.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY