
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
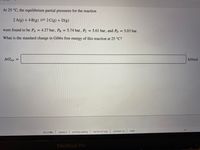
Transcribed Image Text:At 25 °C, the equilibrium partial pressures for the reaction
2 A(g) + 4 B(g)=2C(g) + D(g)
were found to be PA = 4.27 bar, PB = 5.74 bar, Pc = 5.61 bar, and Pp = 5.03 bar.
%3D
%3D
%3D
What is the standard change in Gibbs free energy of this reaction at 25 °C?
AGxn =
kJ/mol
|privacy policy
| contact us
help
about s
careers
terms of use
MacBook Pro
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The reduction of iron(III) oxide (Fe2O3) to pure iron during the first step of steelmaking, 2 FeO3(s) 4Fe(s) + 302(g) is driven by the high-temperature combustion of coke, a purified form of coal: C(s) + O2(g) → CO₂ (g) -> 11 ✓12 Suppose at the temperature of a blast furnace the Gibbs free energies of formation AG, of CO2 and Fe2O3 are -447. kJ/mol and -818. kJ/mol, respectively. Calculate the minimum mass of coke needed to produce 7800. kg of pure iron. Round your answer to 2 significant digits. kg 5arrow_forwardElement X has an enthalpy of fusion (ΔfusH°) of 59.2 kJ mol-1 at its melting point (Tm = 684°C). Calculate ΔSsys for the freezing process,X(l) ⇌ X(s)at 1 bar and 684°C. Express your answer in units of J mol-1 K-1 to 3 significant figures.arrow_forwardIn vacuum distillation, the surrounding pressure inside a chamber is lowered so the solvent boils at a lower temperature. This is useful when isolating compounds that are temperature-sensitive. To what pressure must a chamber be reduced to distill methanol (ΔvapH = 38.56 kJ/mol, TBP = 351 K) at 25.0°C?arrow_forward
- Calculate the change in Gibbs energy for each of the sets of ΔrH∘, ΔrS∘, and T.arrow_forwardThe equilibrium constant for a system at chemical equilibrium at 318 oC is 3.4 x 10-3. Calculate the Gibbs free-energy change, deltaGo, for the system at 318 oC.Enter your answer here: kJ/molarrow_forward7. The vapor pressure of benzene between 10 °C and 30 °C fits the expression 1780 K log()= 7.960- T where T is in K. Calculate (a) the enthalpy of vaporization (Avap H) and (b) the normal boiling point of benzene.arrow_forward
- At 25 °C, the equilibrium partial pressures for the reaction 2 A(g) + 4B(g) = 2C(g) + D(g) were found to be P = 4.62 bar, P = 4.70 bar, Pe=5.81 bar, and Pp = 5.90 bar. What is the standard change in Gibbs free energy of this reaction at 25 C? AG kJ/molarrow_forwardAt 25 °C, the equilibrium partial pressures for the reaction 3 A(g) + 4 B(g) 2C(g) + 2D(g) were found to be PA = 4.67 atm, På = 5.73 atm, Pc = 5.60 atm, and Pp = 5.84 atm. What is the standard change in Gibbs free energy of this reaction at 25 °C? kJ mol privacy policy contact us help terms of use 6:11 PM about us careers 3/25/202arrow_forwardElement X has an enthalpy of fusion of 59.2 kJ mol-1 at its melting point (684°C). Calculate ΔSsys for the process,X(l) → X(s)At 1 bar and 684°C. Express your answer in units of J mol-1 K-1 to 3 significant figures.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY