
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
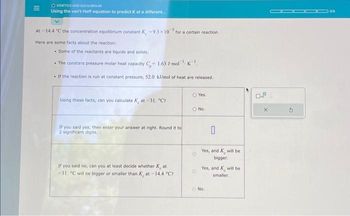
Transcribed Image Text:|||
OKINETICS AND EQUILIBRIUM
Using the van't Hoff equation to predict K at a different.....
At -14.4 "C the concentration equilibrium constant K-9.3x10 for a certain reaction.
Here are some facts about the reaction:
. Some of the reactants are liquids and solids.
• The constant pressure molar heat capacity C,- 1.63 J-mol-K
If the reaction is run at constant pressure, 52.0 kJ/mol of heat are released.
Using these facts, can you calculate Kat-31. "C7
If you said yes, then enter your answer at right. Round it to
2 significant digits.
If you said no, can you at least decide whether Kat
-31. C will be bigger or smaller than Kat-14.4 °C?
O Yes.
O No.
O
0
Yes, and will be
bigger.
Yes, and will be
smaller.
No.
X
2
05
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the final temperature of a reaction that was initially 25 C, given that its equilibrium constant tripled and the reaction’s enthalpy of formation is 60.15 kJ/mol.arrow_forwardConsider the following equilibrium: 2NH, (g) – N, (g) + 3H, (g) AG° = 34. kJ Now suppose a reaction vessel is filled with 5.02 atm of ammonia (NH,) and 9.40 atm of nitrogen (N,) at 159. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of N, tend to rise or fall? fall Is it possible to reverse this tendency adding H2? In other words, if you said the pressure of N, will tend to rise, can that be yes changed to a tendency to fall by adding H,? Similarly, if you said the no pressure of N, will tend to fall, can that be changed to a tendency to rise by adding H2? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H, needed to reverse it. atm Round your answer to 2 significant digits.arrow_forwardConsider the following system at equilibrium where K. = 77.5 and AH° = -108 kJ/mol at 600 K. co (g) + Cl2 (g) = COCI2 (g) The production of COCI, (g) is favored by: Indicate True (I)_or False (E) for each of the following: 1. decreasing the temperature. v 2. increasing the pressure (by changing the volume). 3. decreasing the volume. v 4. removing COCI2 . v 5. removing Cl2 .arrow_forward
- For the reaction 2CH,(g) =C,H2(g) + 3H2(g) Ke = 0.155 at 1611 °C. What is K, for the reaction at this temperature? Express your answer numerically. • View Available Hint(s) ? Kp =arrow_forward2arrow_forwardAmmonia (NH3) decomposes to form nitrogen gas and hydrogen gas with an enthalpy of 89.4kJ/mol. Aclosed container is charged with 6.0 atm ammonia and heated to 25.0°C. At equilibrium the pressure of H2 is3.60atm. a. Write the balanced reaction.arrow_forward
- Nitric oxide reacts with chlorine to form NOCI. 2NO(g) + Cl2g) → 2NOCI(g) The equilibrium constant K at 298K is x 107. (R = 8.314 J/K • mol) Substance: NO(g) Cl2g) NOCIG) AH°F (k]/mol) at 298 K 90.29 51.71 S°U/K• mol) at 298 K 210.65 223.0 261.60arrow_forwardAt -5.92 °C the pressure equilibrium constant K = 5.9 for a certain reaction. P Here are some facts about the reaction: • If the reaction is run at constant pressure, 127. kJ/mol of heat are released. • The constant pressure molar heat capacity C • The net change in moles of gases is -1. = 2.41 J mol -1. K¹. -1 р Yes. ☐ x10 Using these facts, can you calculate K at -27. °C? ○ No. If you said yes, then enter your answer at right. Round it to 2 significant digits. Р If you said no, can you at least decide whether K at -27. °C will be bigger or smaller than K, at −5.92 °C? П Yes, and K, will be р bigger. Yes, and K smaller. will be No. ☑arrow_forwardConsider the reaction: PbCl2(s) → Pb2*(aq) + 2 Cl (aq) AH° = 23.30 kJ/mol and AS° = -12.5 J/K-mol a) When solid PbCl2 is dissolved in water at 25°C, what are the concentrations of Pb2* and Cl at equilibrium? (Hint: Do your ICE chart and Law of Mass Action.) Concentration of Pb2+: mol/L and Concentration of Cl = mol/L (Do not use superscripts, subscripts, or carets. Write 1.2 x 10-3 as 1.2x10-3 or 1.2e-3.) b) At 25°C, This reaction is (reactant or product) favored. Justify your answer with the appropriate calculation on the separate sheet of paper. c) The Gibbs free energy at 25°C, when both the concentrations of the lead ion and chloride ion are 1.2 x 10-³M is kJ/mol. Show your calculations on the separate sheet of paper.arrow_forward
- [Review Topics] [References] Use the References to access important values if needed for this question. Consider the following system at equilibrium where AH° = -111 kJ/mol, and K. = 0.159 , at 723 K. N2 (g) + 3 H, (g)=2 NH3 (g) When 0.21 moles of NH, (g) are added to the equilibrium system at constant temperature: The value of K The value of Qe K. The reaction must O run in the forward direction to restablish cquilibrium. O run in the reverse direction to restablish coquilibrium. O remain the same. It is already at cquilibrium. The concentration of H, will Submit Answer Retry Entire Group 1 more group attempt remainingarrow_forwardConsider the following equilibrium: N₂ (g) + 3H₂(g)2NH₂ (g) AG = -34. KJ 2 Now suppose a reaction vessel is filled with 3.70 atm of hydrogen (H₂) and 8.46 atm of ammonia (NH3) at 1099. °C. Answer the following questions about this system: rise Under these conditions, will the pressure of H ₂ tend to rise or fall? x10 fall Is it possible to reverse this tendency by adding N₂? x Ś ? In other words, if you said the pressure of H₂ will tend to rise, can that be changed to a tendency to fall by adding N₂? Similarly, if you said the yes no pressure of H₂ will tend to fall, can that be changed to a tendency to rise 2 by adding N₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of N₂ needed to reverse it. atm Round your answer to 2 significant digits. ● Oarrow_forwardConsider the following system at equilibrium where K. = 34.5 and AH° = -198 kJ/mol at 1150 K. 2 So, (g) + 02 (g) =2 S03 (g) The production of S03 (g) is favored by: Indicate True (T) or False (F) for each of the following: ]1. decreasing the temperature. v 2. increasing the pressure (by changing the volume). | 3. decreasing the volume. 4. removing SO3 . 5. removing O2 -arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY