
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
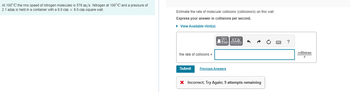
Transcribed Image Text:At 100° C the rms speed of nitrogen molecules is 576 m/s. Nitrogen at 100°C and a pressure of
2.1 atm is held in a container with a 9.0 cm x 9.0 cm square wall.
Estimate the rate of molecular collisions (collisions/s) on this wall.
Express your answer collisions per second.
► View Available Hint(s)
the rate of collisions =
Submit
IVE ΑΣΦ
Previous Answers
•
X Incorrect; Try Again; 9 attempts remaining
collisions
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A 10.0 L container contains a certain gas at a temperature of 25.0◦C and pressure of 9.50 atm. Calculate the number of moles of gas in the container.arrow_forwardn = 3.8 moles of an ideal gas are pumped into a chamber of volume V= (0.083 m³. The initial pressure of the gas is 1.01 × 10° Pa (about 1 atm). What is the initial temperature, in kelvin, of the gas? T = The pressure of the gas is increased ten times. Now what is the temperature, in kelvin, of the gas? T =arrow_forwardCan you solve A and B pleasearrow_forward
- The rms speed of the molecules in 1.2 g of hydrogen gas is 1800 m/s. Part A What is the total translational kinetic energy of the gas molecules? Express your answer with the appropriate units. Etotal = 1.9 kJ Submit ✓ Correct Part B Previous Answers What is the thermal energy of the gas? Express your answer with the appropriate units. Eth = 1944 Submit μA Previous Answers Request Answerarrow_forwardA container contains 0.650 grams of He gas in a volume of 17.5 liter. If 0.260 g of He is remove a constant P and T, what will be the new volume?arrow_forwardThe pressure, volume, and temperature of a mole of an ideal gas are related by the equation PV = 8.31T, where P is measured in kilopascals, V in liters, and T in kelvins. Use differentials to find the approximate change in the pressure if the volume increases from 10 L to 10.6 L and the temperature decreases from 335 K to 330 K. (Note whether the change is positive or negative in your answer. I Round your answer to ti decimal places.arrow_forward
- Two containers of equal volume each hold samples of the same ideal gas. Container A has 2 times as many molecules as container B. If the gas pressure is the same in the two containers, find the ratio of the the absolute temperatures TA and TB ( i.e TA / TB ) . Calculate to 2 decimals.arrow_forwardThe Table shown gives experimental values of the pressure P of a given mass of gas corresponding to various values of the volume V. According to thermodynamic principles, a relationship having the form PVk = C, where k and C are constants, should exist between the variables. Use the natural log (In) and linear regression to solve the following. 1) Find the value of k. a) 0.74 b) 0.86 c) 0.98 d) 1.33 2) Find the value of C. a) 812 b) 765 c) 880 d) 947 3) Estimate P when V = 100.0 in3 a) 18.9 b) 30.8 c) 24.7 d) 49.5 TABLE V(in3) 54.3 61.8 72.4 88.7 118.6 244.9 P(lb/in?) 61.2 49.5 36.6 28.4 19.2 20.1 Write only the letter corresponding to the correct answer.arrow_forwardn = 3.9 moles of an ideal gas are pumped into a chamber of volume V = 0.135 m3 Part (a) The initial pressure of the gas is 1 atm. What is the initial temperature (in K) of the gas? Part (b) The pressure of the gas is increased to 10 atm. Now what is the temperature (in K) of the gasarrow_forward
- Consider a pressure versus volume graph, where the different curves represent different processes done on a gas. Starting at the origin to point 1, the pressure and volume are increased to 2913 Pa and 4.25 m³, respectively. From 1 to 2, the gas expands at constant pressure to a volume of 7.25 m³. From 2 to 3, the pressure rises to 4082 Pa. Finally, from 3 to 4, the gas expands again to 10.85 m³. Rank the curves from the most positive to the most negative amount of work done on the gas. Pressure (Pa) 0 1 3 2 Volume (m³) What is the total work done on the gas? 4 Wo-4 = Most positive work Incorrect 29624.325 23 0 → 1 1 → 2 3→ 4 Most negative work Answer Bankarrow_forwardConsider an ideal gas with an absolute temperature of ?1.T1. To what temperature would the gas need to be heated to double its pressure? Express the answer in terms of ?1.T1. ?2= Consider an ideal gas with a volume of ?1.V1. To what volume would the gas need to be compressed to double its pressure? Express the answer in terms of ?1.V1. ?2=V2=arrow_forwardWhen the gas is in state 1, its temperature is T1. Find the temperature in T3 of the gas when it is in state 3. (Keep in mind that this is an ideal gas.) Express T3 in terms of T1.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON