
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
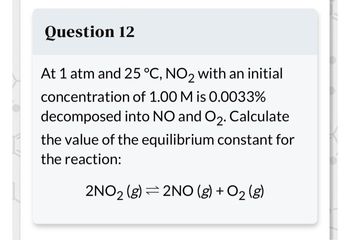
Transcribed Image Text:Question 12
At 1 atm and 25 °C, NO2 with an initial
concentration of 1.00 M is 0.0033%
decomposed into NO and O₂. Calculate
the value of the equilibrium constant for
the reaction:
2NO₂ (g) 2NO(g) + O₂(g)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- One of the steps in the production of sulfuric acid involves the catalytic oxidation of sulfur dioxide. 2 SO2 (g) + O2 (g) → 2 SO3 (g) What is the equilibrium expression?arrow_forwardThe equilibrium constant for the reaction 2 N2(g) + 5 O2(g) ⇆ 2 N2O5(g) was measured at two temperatures: Temperature, K Kp 343 2.5x10−8 944 7.6x10−6 What is ΔHo for the reaction (in kJ/mol)?arrow_forwardCalculate the equilibrium partial pressure of H2arrow_forward
- 15.0 g of glucose reacts with oxygen in a 15.0 L container. At 250°C the system reaches equilibrium. At equilibrium it is determined that there are 5.25 mol of oxygen, 1.84 mol of carbon dioxide and 5.66 mol of steam. Calculate the value of the the equilibrium constant Kc. C6H12O6(s) + 602(g) = 6 CO2(g) + 6H2O(g) O9.64 x 10-5 O5.35 x 10 6 9- O1.87 x 105 O 0.0164 60.9arrow_forwardAmmonia is produced by the Haber process, in which nitrogen and hydrogen are reacted directly using an iron/iron oxide catalyst. For the reaction: N₂(g) + 3H₂(g) → 2NH3(g) AH -92.22 kJ/mol At 300 °C the equilibrium constant for the reaction is 4.34 x 10-³. a) Is this reaction endothermic or exothermic? b) What effect would the following changes have on the equilibrium position. Write forward, reverse or no change: • Add some more nitrogen gas • Increase the volume of the reaction vessel • Decrease the temperature • Remove some hydrogen gasarrow_forwardConsider the following reversible reaction at equilibrium: C6H12O6(aq) + 6 O2(g) 6 CO2(g) + 6 H2O(l). Given that this reaction is exothermic, if heat is added to the equilibrium system, how is the stress relieved?arrow_forward
- What is the correct equilibrium constant expression for the balanced reaction shown below? 2 HCl (g) + I2 (s) 2 HI (g) + Cl2 (g)arrow_forwardGiven the following equation: NH4 (aq) + NO2 (aq) → N2(g) + 2H2O (1) AH = -122.4 kJ/mol How would decreasing the pressure effect the equilibrium? It would cause the equilibrium to shift to the right, favoring the products or the forward reaction. It would cause the equilibrium to shift to the left, favoring the reactants or the reverse reaction. It would not effect the equilibrium.arrow_forwardThe equilibrium constant, Kp, for the following reaction is 0.497 at 500 K. Calculate Ke for this reaction at this temperature. C PC15 (g) → PC13(g) + Cl₂ (9) Kc = ||arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY