
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
if you answered this quesiton before, please dont answer it again please. thanks
use
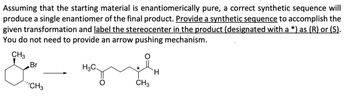
Transcribed Image Text:Assuming that the starting material is enantiomerically pure, a correct synthetic sequence will
produce a single enantiomer of the final product. Provide a synthetic sequence to accomplish the
given transformation and label the stereocenter in the product (designated with a *) as (R) or (S).
You do not need to provide an arrow pushing mechanism.
CH3
Br
"CH3
H3C
*
CH3
H.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which molecule is a complete organic molecule (i.e. all atoms are shown correctly)? A H. c=C–C= C–H H C H. C=C-C-C-H | H. H. C H. C- D H. нн . C С —С—Н C=C–C- C-H H H. ОН Н А I-0-I エーO I-Ú-I I-U-I I-U-I エー○arrow_forwardM Apple Google Disney ESPN Yahoo! Biomedical Careers Program ☆ For the reaction Fe(s) +2HCl(aq)→→→→→FeCl₂(s) + H₂(g) AH° = -7.4 kJ and AS° = 107.9 J/K B Submit Answer Apple ☆ prod03-cnow-owl.cengagenow.com iCloud Yahoo Images Bing Google Wikipedia Facebook Twitter LinkedIn G b COWLv2... b D2L D2L D2L [Review Topics] [References] Use the References to access important values if needed for this question. D2L The maximum amount of work that could be done when 2.08 moles of Fe(s) react at 286 K, 1 atm is Assume that AH° and AS° are independent of temperature. Retry Entire Group 4 more group attempts remaining The Weather Channel C D2L Yelp TripAdvisor M kJ. +arrow_forwardPart: B Nomenclature 5. Write the formula for the following compounds. IUPAC Name a. iron (II) chlorate pentahydrate b. tellurous acid c. lead (II) hypophosphite d. hydroselenic acid e. helium gas f. sodium hypothiosulfite Formula 6. Write the full IUPAC name for each of the following compounds. a. N2F5 b. NH4H2PSO3 c. PbSO2 d. CuTeO2.7H₂O e. Hl (aq) f. HBrO2arrow_forward
- lithium hydrogen sulfite Express your answer as a chemical formula. ΑΣφ A chemical reaction does not occur for this question. Submit Previous Answers Request Answer X Incorrect; Try Again; 5 attempts remaining Part B sodium permanganate Express your answer as a chemical formula. ?arrow_forwardA hydrocarbon contains only the elements O carbon, hydrogen, and nitrogen. carbon and hydrogen. O carbon, hydrogen, and oxygen. O carbon and oxygen.arrow_forwardQuestion 34 of 50 > 0.5 / 1 View POIICIES Show Attempt History Current Attempt in Progress Mercury, water, and bromine are liquids at standard temperature. Their molar entropies are in the sequence H2O < Hg < Br2. Incorrect. Using molecular properties, explain why bromine is more disordered than mercury. Select those that apply. Bromine is diatomic. Mercury is monoatomic. Diatomics are more ordered than monoatomics. Water has strong hydrogen bonding interactions. O Hydrogen-bonded substances are less ordered than substances without hydrogen-bonds.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY