
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
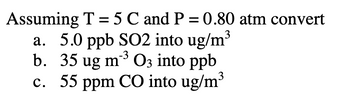
Transcribed Image Text:Assuming T = 5 C and P = 0.80 atm convert
a. 5.0 ppb SO2 into ug/m³
-3
b. 35 ug m²³ O3 into ppb
c. 55 ppm CO into ug/m³
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please answerarrow_forward4. At 5°C and 1 atm, x L of O2 gas is required to react with 0.51 g of glucose, what is the value of x? The unbalanced reaction is shown below: C6H12O6 (s) + O2(g) → CO2(g) + H2O(l)arrow_forwardA student experimentally determines the gas law constant, R, by reacting a small piece of magnesium with excess hydrochloric acid and then collecting the hydrogen gas over water in a eudiometer. Based L-atm on experimentally collected data, the student calculates R to equal 0.0832 mol·K L-atm Ideal gas law constant from literature: 0.08206 mol·K (a) Determine the percent error for the student's R-value. Percent error = % (b) For the statements below, identify the possible source(s) of error for this student's trial. The student notices a large air bubble in the eudiometer after collecting the hydrogen gas, but does not dislodge it. The student does not clean the zinc metal with sand paper. The student does not equilibrate the water levels within the eudiometer and the beaker at the end of the reaction. The water level in the eudiometer is 1-inch above the water level in the beaker. The student uses the barometric pressure for the lab to calculate R.arrow_forward
- Please help mearrow_forward9:08 PM ☹ & = ikf MB² = 5.12 °C/m 19²2- (-130%) = AT £ 1Jf=18492 ug°c = (5.12 L/m) n = 3.611 ●●● 25 Question 9 of 14 | g 1 3 4 6 7 9 +/- 0 Tap here or pull up for additional resources 8 What mass in grams of NaCl would need to be added to 2051 g of water to increase the boiling temperature of the solution by 1.500 °C? (Kb for water is 0.5100 °C/m) X C 20% 0 Submit x 100arrow_forwardIn the fermentation of sucrose Calculate how many milliliters of CO2 (at STP) would be produced assuming all of the sucrose were consumed during fermentation. with 10g of sucrose.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY