
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
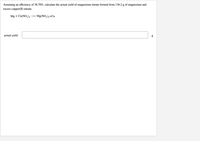
Transcribed Image Text:Assuming an efficiency of 38.70%, calculate the actual yield of magnesium nitrate formed from 136.2 g of magnesium and
excess copper(II) nitrate.
Mg + Cu(NO,),
Mg(NO,),+Cu
actual yield:
Expert Solution
arrow_forward
Step 1
The given chemical equation is as follows:
The efficiency of the reaction = 38.70 %
The given mass of Mg = 136.2 g
The actual yield of the =?
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Assuming an efficiency of 44.10%, calculate the actual yield of magnesium nitrate formed from 141.6 g of magnesium and excess copper(II) nitrate. Mg + Cu(NO3)2 → Mg(NO,),+Cu actual yield: Show Allarrow_forwardAssuming an efficiency of 32.70%,32.70%, calculate the actual yield of magnesium nitrate formed from 145.4 g145.4 g of magnesium and excess copper(II) nitrate. Mg+Cu(NO3)2⟶Mg(NO3)2+Cuarrow_forwardFor each reaction, write the chemical formulae of the oxidized reactants in the space provided. Write the chemical formulae of the reduced reactants in the space provided. reactants oxidized: CuCl, (aq) + Mg(s) - MGCL, (aq) + Cu(s) reactants reduced: A ? reactants oxidized: Feso, (aq) + Zn(s) Fe(s) + ZnSO, (aq) reactants reduced: reactants oxidized: 2AGNO, (aq) + Mg(s) 2Ag(s) + Mg(NO,), (ag) → reactants reduced:arrow_forward
- Assuming an efficiency of 39.90%,39.90%, calculate the actual yield of magnesium nitrate formed from 145.7 g145.7 g of magnesium and excess copper(II) nitrate. Mg+Cu(NO3)2⟶Mg(NO3)2+Cuarrow_forwardAssuming an efficiency of 28.00%,28.00%, calculate the actual yield of magnesium nitrate formed from 133.6 g133.6 g of magnesium and excess copper(II) nitrate. Mg+Cu(NO3)2⟶Mg(NO3)2+Cu Actual yield in gramsarrow_forwardConsider the reaction: NO,(g) + CO(g) → NO(g) + CO,(g) How many electrons are transferred in this reaction?arrow_forward
- Lithium batteries are used throughout our modern world – in cell phones, laptops, tablets, etc. In fact, if you are regularly recharging your device, it’s highly likely it contains a lithium ion battery. The lithium iron phosphate battery provides stable function over time. The overall reaction for the lithium iron phosphate battery is below: LiFePO4 + 6 C -> LiC6 + FePO4 A certain lithium battery weighs 0.6 g and has a capacity of 2250. mA hr (that is, the cell can store charge equivalent to a current of 2250. mA flowing for 1 hr). What is the capacity of this cell in coulombs? What mass of the reactants is needed to deliver 2250. mA hr? What percentage of the cell mass consists of reactants?arrow_forwardIn a redox reaction, electrons are transferred from the substance being oxidized to the reducing agent. O from the oxidizing agent to the reducing agent. from the substance being reduced to the oxidizing agent. from the substance being oxidized to the substance being reduced. O from the substance being reduced to the substance being oxidized DOO00arrow_forwardAssuming an efficiency of 43.70%, calculate the actual yield of magnesium nitrate formed from 110.4 g of magnesium and excess copper(II) nitrate. Mg + Cu(NO3)2 actual yield: Mg(NO3)₂ + Cu garrow_forward
- How many total electrons are transferred during the reaction of the oxidation of oxidation according to the following reaction? C(s) + O2(g) --> CO2(s)arrow_forwardConsider the following unbalanced oxidation-reduction reaction: CO(g) + I2O5(s) --> CO2(g) + I2 (s) how many electrons are transferred in this reaction if it is balanced? 4 6 5 8 10arrow_forwardWhich of the following is an oxidation reduction (redox) reaction? 1. MgCl2 (aq) + Pb(NO3)2 (aq) = Mg(NO3)2 (aq) + PbCl2(s) 2. 2 Zn (s) + FeCl2 (aq) = Fe (s) + 2 ZnCl2 (aq) 3. 2 H2SO4 (aq) + NaOH (s) = 2H2O (l) + Na2SO4 (aq) 4. Na3PO4 (aq) + FeCl3 (aq) = 3NaCl (aq) + FePO4 (s)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY