
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
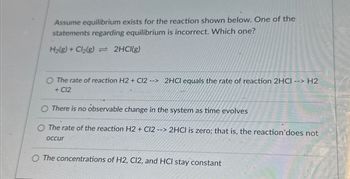
Transcribed Image Text:Assume equilibrium exists for the reaction shown below. One of the
statements regarding equilibrium is incorrect. Which one?
H₂(g) + Cl₂(g) = 2HCl(g)
O The rate of reaction H2 + Cl2 --> 2HCl equals the rate of reaction 2HCI --> H2
+ C12
There is no observable change in the system as time evolves
O The rate of the reaction H2 + C12 --> 2HCl is zero; that is, the reaction'does not
occur
The concentrations of H2, C12, and HCI stay constant
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- = O KINETICS AND EQUILIBRIUM Using Le Chatelier's Principle to predict the result of changing... Acetylene and oxygen react to form carbon dioxide and water, like this: 2C₂H₂(g) +50₂(g) → 4CO₂ (g)+2H₂O(g) Suppose a mixture of C₂H₂, O₂, CO₂ and H₂O has come to equilibrium in a closed reaction vessel. Predict what change, if any, the perturbations in the table below will cause in the composition of the mixture in the vessel. Also decide whether the equilibrium shifts to the right or left. perturbation Some H₂O is removed. Some C₂H, is added. change in composition The pressure of C₂H₂ will The pressure of O₂ will The pressure of O₂ will The pressure of CO₂ will ? ? ? ? ? V go up. go down. not change. shift in equilibrium O to the right to the left x O (none) 3/5 to the right to the left (none)arrow_forwardThe reaction below was allowed to react at 375 degrees Celcius until it formed an equilibrium. At equilibrium, the 16.2 L container was found to contain 1.9 mol SiF4, 1.1 mol H2O, 3.5 mol SiO2, and 2.9 mol HF. What is the Kc of the reaction at this temperature? SiF4 (g) + 2H2O (g) <--> SiO2 (s) + 4HF(g)arrow_forwardThe progress of the reaction H2(g) + Br2(g) 2 2 HBr(g) can be monitored visually by following changes in the color of the reaction mixture (Br2 is reddish brown, and H2 and HBr are colorless). A gas mixture is prepared at 700 K, in which 0.40 atm is the initial partial pressure of both H2 and Br, and 0.90 atm is the initial partial pressure of HBr. The color of this mixture then fades as the reaction progresses toward equilibrium. Give a condition that must be satisfied by the equilibrium constant K (for example, it must be greater than or smaller than a given number).arrow_forward
- 5. Study the following reactions: 2H2O2⇌2H2O+O22H2O2⇌catalyst2H2O+O2Reaction 1: uncatalyzedReaction 2: catalyzed If the two reactions occur at the same temperature, which statements are true? Select all that apply. The catalyzed reaction will reach equilibrium faster than the uncatalyzed reaction. The uncatalyzed reaction will reach equilibrium faster than the catalyzed reaction. The equilibrium position of the catalyzed reaction is greater than that of Reaction 1. The equilibrium position is the same in both the catalyzed reaction and the uncatalyzed reaction. The equilibrium position of the catalyzed reaction is shifted toward the products side. The equilibrium position of the catalyzed reaction is shifted toward the reactants side.arrow_forwardConsider the reaction for the decomposition of hydrogen disulfide: 2H2S(g)----> 2H2(g)+S2 kc= 1.67*10^-7 @ 800°C The reaction is carried out at the same temperature with the following initial concentrations: [H2S]= 4.50*10^-4 M [H2]= 0 M [S2]= 0 M Find the EQUILIBRIUM concentration of S2:arrow_forward+ Imagine 212. mmol of HCH,CO, are removed from a flask containing a mixture of HCH,CO,, H,O, H,O' and CH,CO, at equilibrium, and then answer the following questions. Zero. What is the rate of the forward Greater than zero, but less than the rate of the reverse reaction. reaction before any HCH3CO2 has been removed from the flask? Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction. Zero. What is the rate of the forward Greater than zero, but less than the rate of the reverse reaction. reaction just after the HCH3CO2 has been removed from the flask? Greater than zero, and equal to the rate of the reverse reaction. Greater than zero, and greater than the rate of the reverse reaction. Zero. What is the rate of the forward Greater than zero, but less than the rate of the reverse reaction. reaction when the system has again reached equilibrium? Greater than zero, and equal to the rate of the reverse…arrow_forward
- Please don't provide handwriting solutionarrow_forwardpart b pleasearrow_forward*** S 19. Solid ammonium hydrogen sulfide is introduced into a 2.00-L flask, and the flask is şealed. If this solid decomposes according to the equation below DAUS Ca Nothange NH4HS(s) NH3(g) + H₂S(g), Kp = 0.108 at 25°C, what is the minimum mass of ammonium hydrogen sulfide that must be present in the flask initially if equilibrium is to be established at 25°C? A) 0.917 g B) 1.37 g C) 2.74 g D) 0.581 g E) 0.452 garrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY