
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please provide an explanation with the answer
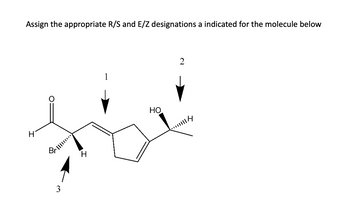
Transcribed Image Text:Assign the appropriate R/S and E/Z designations a indicated for the molecule below
Η
○
PIIIIII...)
Bri
3
1
2
HO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- #9. Use the information provided below to find out the molecular formula and structure of the compound. QE-300 CDCl3 al Relative Intensity 100 80 60 40 20 +0 0-min 10 3454 #1 3456 57 2907 15 2944 20 2910 36 2879 50 TO ALLE 1 In 20 30 2716 81 2001 04 2438 1 2154 84 1991 84 1972 77 1733 4 1476 23 1470 25 1447 19 1421 16 1116 25 1160 9 40 50 1306 1270 1140 1022 9 974 27 657 25 76466 6 $ 60 m/z MENYERL 70 722 27 608 13 1900 80 90 100 110 120arrow_forward(b) 0.23 MMgSO4 lons: SO2- O Mg2+ O4 O Mg* So+ O SO,2- masscation i massanion %3Darrow_forwardYou measured the carbon-carbon bond lengths of four different compounds in Experiment 8 (Molecular Modeling and IR spectroscopy). Which of the following compounds has the shortest average C-C bond length? Benzene Cyclohexene Cyclohexane O Cyclohexadiene Which of the following statement(s) is(are) true? Select all that apply: When two atoms are bonded together, the distance between atoms is the same all the time. In the IR spectrum, carbonyl groups (C=O) have strong sharp peaks ranging from 1700 cm 1 to 1750 cm-1. When a molecule absorbs an IR radiation, electrons are excited within the molecule and each absorbed electron creates a band in the molecules IR spectrum. IR spectroscopy is useful in confirming the structure of the analyzed compound. Molecule N2 does not absorb IR radiationarrow_forward
- Halogenated compounds are particularly easy to identify by their mass spectra because both chlorine and bromine occur naturally as mixtures of two abundant isotopes. Recall that chlorine occurs as 35Cl (75.8%) and 37Cl (24.2%); and bromine occurs as 79Br (50.7%) and 81Br (49.3%). At what masses do the molecular ions occur for the following formulas? What are the relative percentages of each molecular ion? (a) Bromomethane, CH3Br (b) 1-Chlorohexane, C6H13Clarrow_forwardWhy do aldehydes, esters, and amides all have a strong absorption in the 1630-1780 cm1 region of their IR spectra? A) The bond between H and the sp³-hybridized C in these functional groups vibrates in this energy range. B) Each of these functional groups has at least two resonance structures, and the different vibrations of the resonance structures give off energy in this region. C) The bond between O and the sp²-hybridized C in these functional groups vibrates at a frequency in this energy range. D) Light at this wavenumber causes the average C to O bond length to increase which causes more of this light to be transmitted. E) An electron in the bond of these functional groups gets excited to the * orbital.arrow_forwardWhich of the following molecules is represented in this IR spectrum? INFRARED SPECTRUM TRANSMITTANCE 0.8 0.6 0.4 0.2 3000 Wavenumber (cm-1) NIST Chemistry WebBook (http://webbook.nist.gov/chemistry) B 2000 -NH₂ CH3 OH с 1000 D OHarrow_forward
- 15) Chemical Formula: CsH8O₂ 5x2=10+2=12-8=4/2=2. IR: strong peak 1720cm-¹, 1650cm-1( hard to see but, all peaks that integrate to 1H are doublet of doublets) 5 1H 1H 1 3 3H PPM 3H −2 0arrow_forwardThe leaves of the Brazilian Tree Senna multijunga contain a number of pryidine alkaloids that inhibit acetylcholinterinase. Two recentyl isolated isomeric compounds have the strcture have the strcture shown below. (NOTE: M=293) Use the mass spectral data provided to determine the precise location of the hydroxyl group in each isomer. Isomer A: EI-MS, m/z(rel. int): 222(20), 150(10), 136(25), 123(100) Isomer B:EI-MS, m/z(re;. int): 236(20), 150(10), 136(25), 123(100)arrow_forwardBelow are two molecules, cyclohexylamine (A) and aniline (B). When analyzed by Infrared Spectroscopy (IR) two different frequencies are observed for the C-N absorption band. Which molecule will have the lower wavenumber absorption band for the C-N bond. Explain why in 35 words or less. „NH2 NH2 А Barrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning