
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
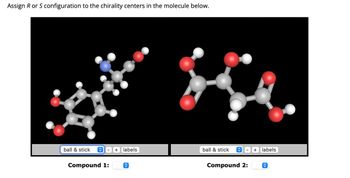
Transcribed Image Text:Assign R or S configuration to the chirality centers in the molecule below.
ball & stick
+ labels
ball & stick
+
labels
Compound 1:
Compound 2:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following properties differs for two enantiomers? A) boiling point B) melting point Question 5 of 14 C) interaction with plane-polarized light D) solubility E) vapor pressure Submit +arrow_forwardH- HO HO- HO OH H H OH H -H H CH₂OH Draw four different horizontal zig-zag bond line structures that correctly represent the Fischer projection of 2,3,4,5,6-pentahydroxyhexanal shown below. One of your bond-line structures should have the carbonyl on the left hand side of the molecule pointing up, another on the left side pointing down, another on the right side pointing up, another on the right side pointing down (the terminal CH₂OH is marked in blue) Also, assign each chirality center as (R) or (S) in the original Fischer projection (you only have to do this once as it's the same for all depictions of the molecule). H OH ОН. H H OHarrow_forwardANSWER THE FOLLOWING PROBLEM AND EXPLAIN YOUR ANSWER FOR BETTER UNDERSTANDINGarrow_forward
- Hw 1.arrow_forwardHow many chiral centers are in the following compound and how many stereoisomers are there? Но -HO- 3 chiral centers, 6 stereoisomers O 2 chiral centers, 4 stereoisomers O 3 chiral centers, 4 stereoisomers O 2 chiral centers, 3 stereoisomersarrow_forwardDetermine the configuration of each stereogenic center in the compound below. Highlight the carbon in red if the configuration is R, and in blue if the configuration is S. Br HO 0 3arrow_forward
- Assign the absolute configuration (R/S assignments) at each chirality center:C1:C2:C3:arrow_forwardDoes the molecule below exist as a pair of enantiomers? If so, change the bonds to wedges and dashes to reflect R stereochemistry. If the molecule does not exist as a pair of enantiomers, check the box below. Does not exist as enantiomers Cl CH3 OR Xarrow_forwardDetermine if the following compound has R or S chirality. OH но None ORarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY