
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Assign E or Z to the following
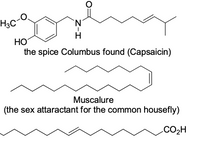
Transcribed Image Text:H3C
HO
the spice Columbus found (Capsaicin)
Muscalure
(the
sex attaractant for the common housefly)
CO2H
Z-I
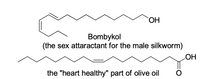
Transcribed Image Text:HO
Bombykol
sex attaractant for the male silkworm)
(the
HO
the "heart healthy" part of olive oil
Expert Solution
arrow_forward
Step 1
The isomers which have a restricted rotation around the double bond is known as geometrical isomers.
The isomers which have different spatial arrangements are known as cis- trans isomers.
In cis isomers; the functional groups are present on the same side of double bond while in trans isomers; the functional groups are present on the opposite side of double bond.
According to CIP rule; higher atomic number take the first priority for assigning the cis trans configuration than lower atomic number.
Step by stepSolved in 6 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How to figure out E and Z fornalkenes?arrow_forwardKelerences Keview topic Draw a structural formula for the major product of the reaction shown. Cl2 H20 CH;CH2CH;CH=CHz • Show product stereochemistry IF the reactant alkene has both carbons of the double bond within a ring. Do not show stereochemistry in other cases. • If the reaction produces a racemic mixture, just draw one stereoisomer. 2reg 2reg M) ots 2reg pts 2req 1 pts 2req 1 pts 2reg ...1 pts ChemDoodle Previous Next> Save and Exit APR tv MacBook Air 80 DII DD F3 F4 FS F6 F7 F8 F10 F1 F12 #3 $ & + 3 4 6. 7 8 %3D dele E Y | { } P [ F G H. J K C V N M ? .. .. * C0 Barrow_forwardRank the alkenes in order of decreasing heat of hydrogenation. A Highest heat of hydrogenation Lowest heat of hydrogenation Answer Bankarrow_forward
- An alkene having the molecular formula C,H12 is treated sequentially with ozone (O3) and zinc/acetic acid to give the product/s shown. CH3CH2CH,CH½CH2CH Draw a structural formula for the alkene. You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. P opy aste Previous Nextarrow_forward8. Name the following alkenes. Don't forget to assign E/Z nomenclature. each) maarrow_forward4. The enthalpies, AH°, for the hydrogenation of three alkenes are shown below: Alkene A: -50 kcal/mole Alkene B: -60 kcal/mole Alkene C: -70 kcal/mole Which alkene is the least stable and why?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY