
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Assign a hybridization to each interior atom in guanine.
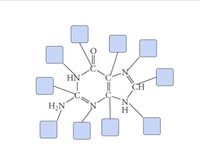
Transcribed Image Text:HN
CH
H,N
H
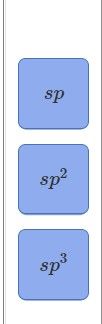
Transcribed Image Text:ds
sp?
sp3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the molecule shown below, identify the hybridization on the C atom with the double bonded oxygen and the approximate bond angle labeled beta: Group of answer choices hybridization: sp2 bond angle: 120° hybridization: sp3 bond angle: 109.5° hybridization: sp3d bond angle: 90° hybridization: sp3 bond angle: 120°arrow_forwardTwo isomers share the molecular formula C3H4. In Structure A two ofthe carbon atoms are sp hybridized and one carbon atom is sp3 hybridized. In Structure B, two of the carbon atoms are sp2 hybridized and one carbon atom is sphybridized. b)Draw “3D”-looking structures on paper, make a sketch of each of the isomers. (This should be a sketch of themolecules, not diagrams of overlapping orbitals!) Your drawing should show thecorrect molecular geometry around each carbon atom. [HINT: NEITHER of thesemolecules is planar!!! To draw them correctly, you will have to consider why!]arrow_forward1. Concept map: For carbon, draw out the orbital energy diagram of sp³, sp², and sp hybridized orbitals, including the leftover unhybridized orbitals, if any.arrow_forward
- Describe each highlighted bond in terms of the overlap of atomic orbitals. (If the highlighted bond is not a pi bond, select the blank option from the dropdown menu.)arrow_forwardFor each of the following condensed structures: (i) draw the corresponding Lewis structures and (ii) provide the hybridization to all carbon atoms; (iii) draw individual p orbitals for all the π bonds with directions clearly indicated. a) CH2CHCHCH2 b) CH3CCCH3 c) CH2CCHCH3arrow_forwardPlease help, a bit conufsedarrow_forward
- 2) a) Consider the following molecule . Given what you have learned about hybridization theory, draw an image or images explaining the bonding situation in this molecule. I want you to draw out all of the orbitals, hybrid orbitals and how they overlap to form the bonds in the molecule. Indicate the % s or p character in the given atomic and hybrid orbitals. Which C-C bond or bonds are the longest? In a paragraph or so explain the image or images you just drew. b) Lastly, consider the molecule below. Indicate the Molecular formula, the molar mass, label the hybridization of each atom except for hydrogen, indicate any chiral centers with a *, which bond or bonds are the shortest, identify by name of each functional group with an arrow pointing to the group.arrow_forwarda) Consider a molecule of acetone cyanohydrin, shown below. What is the hybridization and molecular geometry at CA, CB, and O? (b) For Carbon B (CB) and nitrogen (N), draw orbital energy diagrams showing the orbitals involved in bonding after hybridization. Be sure to fill the orbitals appropriately with valence electrons. c) In your diagrams in part (b), which orbitals are involved in sigma and pi bonding? Orbitals Involved in Sigma Bonding Orbitals Involved in Pi Bonding 2p sp sp2 sp3 2p sp sp2 sp3 (d) In a sample containing many molecules of acetone cyanohydrin (and no other substances), what type(s) of intermolecular forces could be present? Choose all that apply. Covalent Bonds Hydrogen Bonds Dipole-Dipole Forces London Dispersion Forcesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY