
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
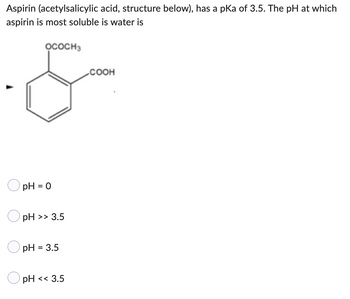
Transcribed Image Text:Aspirin (acetylsalicylic acid, structure below), has a pKa of 3.5. The pH at which
aspirin is most soluble is water is
OCOCH3
COOH
pH = 0
pH >> 3.5
pH = 3.5
pH << 3.5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- You are preparing 1 Lt of TAE (Tris Acetate EDTA) gel running buffer from a 50x stock solution. How much 50 x TAE and how much water will you combine?arrow_forwardHow many moles of Nal would be found in 250mL of a 4.5 M solution? Write your answer with three digits beyond the decimal point (IMPORTANT - zeroes count as a digit) The answer is molesarrow_forward0.03 The uv spectrum of a protein solution shows A280 nm = 0.43 and A260 nm = What is the approximate protein concentration in mg/mL?arrow_forward
- Make a 100 ml of a 1M stock solution of NaCl (you will need the molecular weight of NaCl).arrow_forwardHow would I calculate the amount of each component in each solution? Stock solutions provided: 0.5 M EDTA, pH 8 10% bromophenol blue 10% xylene cyanol Sucrose powder Tris powder Glacial acetic acid Solution A: 50 mL of 50x Tris Acetate EDTA (TAE) Buffer 2M Tris Base 50mM EDTA, pH 8 11.4% (v/v) glacial acetic acid Solution B: 1L of 1x TAE Buffer 40mM Tris-acetate 1mM EDTA Solution C: 5mL of 10x Loading Dye 0.25% (w/v) bromophenol blue 0.25% (w/v) xylene cyanol 66.5% (w/v) sucrose 100mM EDTA, pH 8arrow_forwardyou get an order of 5% amino acid 15% dextrose premixed parenteral nutrition solution, 2L at 83mls/hr. Your pharmacy technician tells you there is a manufacture's backorder on those. How many ml of 20% dextrose would you need to provide the same amount of dextrose in 24 hours?arrow_forward
- Calculate the unknown concentration of the PROTEIN C with an absorbance value of A412 given the standard curve indicated in the table below. Write your final answer (NUMBER ONLY) in two decimal places rounded off. Protein concentration (µg/mL) 0 0.02 0.04 0.06 0.08 0.10 Your answer APIENT Absorbance 0.000 0.161 0.284 0.438 0.572 0.762arrow_forwardwhat is the pH of a solution with a H+ concentration of 1.44 x 10-3 Marrow_forwardBonlyarrow_forward
- Protein Solubility 3.5 5.5 5 5 0.068 0.028 Absorbance 0.098 Conc.(mg/ml) 0.195 0.130 0.044 0.9% 2.786% 9.429% 11.3: % solubility 4.179% The above table indicates the concentration of protein in the diluted supernatant and the supernatant before dilution at different pH. Dilution Factor 30 22.5 15 PH 7.5 1.5 4.5 5 0.027 3 0.042 Protein Solubility Versus pH 6.5 6 4.5 pH Value The above figure shows a plot of protein solubility versus pH. 50 7.5 7.5 50 0.032 0.053 8.5 50 0.054 0.100 21.429% 9 Please provide a brief discussion and explanation of the results. (using isoelectric point and net charge to explain)arrow_forwardA buffer contains 0.015 mol of lactic acid (pK₁ = 3.86) and 0.080 mol of sodium lactate per liter. H₂C OH Lactic acid OH Calculate the pH of the buffer. H₂C. Calculate the change in pH after adding 9.0 mL of 0.10 M HCl to 1.0 L of the buffer. O OH Sodium lactate Calculate the change in pH after adding 9.0 mL of 0.10 M HCl to 1.0 L of pure water. O Na+ buffer pH: buffer pH change: water pH change: units unitsarrow_forwardpH of solution 14.00 12.00 10.00 8.00 6.00 First 4.00 equivalence point 2.00 0 First midpoint Second equivalence point Third midpoint pH = pKa=12.32 Third equivalence point HPO4(aq) + OH(aq) PO4(aq) + H2O(1) Second midpoint pH = pKa = 7.21 H2PO4 (aq) + OH(aq) HPO42 (aq) + H2O(1) Using the Henderson- Hasselback equation, show how to create 2L of a 0.1 M KPhos pH 7.5 buffer using K2HPO4 and KH2PO4. The chart to the left should help you understand what pKa to start with. Show your work. pH = pKa = 2.16 H3PO4(aq) + OH(aq) H2PO4(aq) + H2O(l) 25.0 50.0 75.0 100.0 Volume of NaOH added (mL)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON