
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please, I do not understand this question. Steps leading to the CORRECT answer would be helpful!
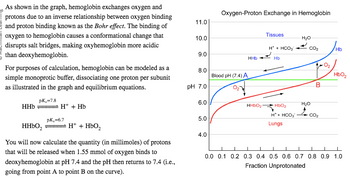
Transcribed Image Text:As shown in the graph, hemoglobin exchanges oxygen and
protons due to an inverse relationship between oxygen binding
and proton binding known as the Bohr effect. The binding of
oxygen to hemoglobin causes a conformational change that
disrupts salt bridges, making oxyhemoglobin more acidic
than deoxyhemoglobin.
For purposes of calculation, hemoglobin can be modeled as a
simple monoprotic buffer, dissociating one proton per subunit
as illustrated in the graph and equilibrium equations.
pK₁=7.8
HHb
H+ + Hb
pK₁=6.7
HHbO₂
H+ + HbO₂
You will now calculate the quantity (in millimoles) of protons
that will be released when 1.55 mmol of oxygen binds to
deoxyhemoglobin at pH 7.4 and the pH then returns to 7.4 (i.e.,
going from point A to point B on the curve).
11.0
10.0
9.0
8.0
pH 7.0
6.0
5.0
4.0
Oxygen-Proton Exchange in Hemoglobin
Blood pH (7.4) A
HHb
HHbO₂
Tissues
H+ + HCO3
Hb
HbO₂
H* + HCO3
Lungs
H₂O
CO₂
H₂O
B
CO₂
Hb
HbO₂
0.0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0
Fraction Unprotonated
![Step 1: Calculate the ratio of [HHb] to [Hb] at pH 7.4.
Step 2: Calculate the millimoles of HHb in 1.55 mmol
deoxyhemoglobin at pH 7.4.
Step 3: Calculate the ratio of [HHbO₂] to [HbO₂] at pH 7.4.
Step 4: Calculate the millimoles of HHbO₂ in 1.55 mmol of
oxyhemoglobin at pH 7.4.
Step 5: Calculate the millimoles of protons that will be
released when 1.55 mmol of oxygen binds to
deoxyhemoglobin at pH 7.4.
[HHb]
[Hb]
иннь =
[HHbO₂]
[HbO₂]
иHHbO₂ =
nprotons =
2.51
2.0002
0.2
0.4648
1.54
mmol
mmol
mmol](https://content.bartleby.com/qna-images/question/25d5c273-ce26-424b-bd36-a8a6b930ab8d/22374f50-40f8-4aa1-8221-4c97f43e0bb1/o7tprgu_thumbnail.png)
Transcribed Image Text:Step 1: Calculate the ratio of [HHb] to [Hb] at pH 7.4.
Step 2: Calculate the millimoles of HHb in 1.55 mmol
deoxyhemoglobin at pH 7.4.
Step 3: Calculate the ratio of [HHbO₂] to [HbO₂] at pH 7.4.
Step 4: Calculate the millimoles of HHbO₂ in 1.55 mmol of
oxyhemoglobin at pH 7.4.
Step 5: Calculate the millimoles of protons that will be
released when 1.55 mmol of oxygen binds to
deoxyhemoglobin at pH 7.4.
[HHb]
[Hb]
иннь =
[HHbO₂]
[HbO₂]
иHHbO₂ =
nprotons =
2.51
2.0002
0.2
0.4648
1.54
mmol
mmol
mmol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Complete the table below. Round each of your entries to 2 significant digits. You may assume the temperature is 25 °C. conjugate acid formula 0 0 ΗΝΟ, Ka 0 0 4.5 X 10 4 conjugate base Kb formula CH,NH, HCO3 П 4.4 X 10 2.2 × 10 0 4 8 90 X S x10arrow_forwardAnswer 3 and 4arrow_forwardDraw the skeletal structure of the product(s) for the Lewis acid-base reaction.arrow_forward
- Calculate the pH of a 0.10 mol L- solution of hydrazine, N2H4. KŁ for hydrazine is 1.3 x 10-6, Express your answer numerically using two decimal places. • View Available Hint(s) pH = Submit Part E Calculate the pH of a 0.10 mol L-1 solution of hypochlorous acid, HOCI. Ka of HOCI is 3.5 × 108. Express your answer numerically using two decimal places. • View Available Hint(s) ? pH =arrow_forwardQUESTION 2 What is the pH of a solution with OH- of 0.0001 M KOH at 25°C. Give the answer in 2 significant figures. State whether the solution is an acid, base, or neutral. QUESTION 3 Fruit, milk, and wheat are examples of: O a. Nucleic Acids O b. Lipids O c. Carbohydrate O d. Proteinsarrow_forwardA solution containing 12.6 grams sodium hydrogen phosphate and 15.7 grams of sodium phosphate. find the pH of the solution.arrow_forward
- All of the following statements about the pH scale are true EXCEPT O Cola drinks are very acidic. O The difference in concentration between pH10 and pH 12 is 20. O Neutral is pH 7.00 O Blood is slightly basic. O pH 2 is a strong acid.arrow_forwardStudents in a chemistry lab were using the 2 chemicals below in an experiment. Chemical pH Chemical #1 12 Chemical #2 Susan believes that Chemical #1 is a strong acid and that Chemical #2 is a strong base. Andrew believes that Chemical #1 is a strong base and that Chemical #2 is a strong acid. Which student is correct?arrow_forwardFor some questions you will need to use the special periodic table attached in the images! Treat Je, Qu, Ap, and Bg as NONMETALS! Classify each of the following alcohols as primary (1°), secondary (2°), or tertiary (3°)? Look at attached images (Please show work so I can understand going forward)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY