
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
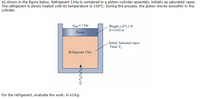
Transcribed Image Text:As shown in the figure below, Refrigerant 134a is contained in a piston-cylinder assembly, initially as saturated vapor.
The refrigerant is slowly heated until its temperature is 150°C. During the process, the piston moves smoothly in the
cylinder.
Weight 471.1 N
D= 0.02 m
Patm=I bar
3D
Piston
Initial: Saturated vapor
Final: T2
Refrigerant 134a
For the refrigerant, evaluate the work, in kJ/kg.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- By actual measurement, the enthalpy of steam at 8 bar is found to be 3000 KJ/Kg. What is the quality of steam? If 500KJ/Kg of heat is added to this steam, what is the (i) Superheated temperature (ii) Degree of superheat (iii) Enthalpy of superheat?arrow_forward4. One mole of helium gas is injected into each side of a slidable, airtight lead piston that separates two chambers of a sealed cylinder. The outside of the cylinder is insulated everywhere except where noted below. The cylinder and piston have lengths and cross- sectional area labeled in the diagram. The helium in the left chamber is heated from outside at a rate of 450 W, and the helium in the other chamber expels heat into a cool region. The full system eventually comes to steady-state. When it reaches steady state, the force needed to hold on the end cap is measured to be 18,000 N, and the piston has slid to an equilibrium point that is a distance à from the left end. force holding cap = 18,000N pressure. cross-sectional area of cylinder = 0.02m² x = ? Imol He do dt = +450W 45cm 15cm lead piston a. Find the temperature difference of the two chambers. b. Find the distance x. dQ dt Assume helium behaves as an ideal gas, and that heat transfer through the container walls is…arrow_forward4. At a pressure of 900 kPa, the specific enthalpy is 2000 kJ/kg. What is the phase of the water? a. Solid b. Solid-liquid mixture c. Subcooled liquid d. Saturated liquid e. Saturated mixture f. Saturated vapor g. Superheated vapor For the water in problem 4, determine the following: a. If saturated mixture calculate the quality, x = b. Determine the specific internal energy.arrow_forward
- ! Required information NOTE: This is a multi-part question. Once an answer is submitted, you will be unable to return to this part. A piston-cylinder device initially contains 1 kg saturated liquid water at 200°C. Now heat is transferred to the water until the volume quadruples and the cylinder contains saturated vapor only. The saturated liquid properties of water at 200°C 3 are vf= 0.001157 m³/kg and uf- 850.46 kJ/kg (Table A-4). Water mkg 200°C Q Determine the final temperature and pressure. (You must provide an answer before moving to the next part) The final temperature is The final pressure is °C. kPa.arrow_forwardDetermine the quality of 290 kPa of steam when 570 kJ / kg of energy is lost from the saturated vapor.a. steam quality = ......%b. what is the temperature of the steam = ..... Carrow_forwardN = 0 An insulated tank of volume 10+(10+N)/25 m and with a valve was filled with perfect tri- atomic gas at pressure 10 bar, where N is equal to the last two digitals in your student's ID number. Initially the valve was closed. A paddle wheel was mounted inside the tank and was run by 20 kW engine. The paddle wheel was turn on and at the same time the exit valve was opened. The gas temperature was constant though the whole process (due to very slow flow) and equal to 300 K. Calculate the time needed to decrease the pressure in the tank to 5 bar.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY