
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
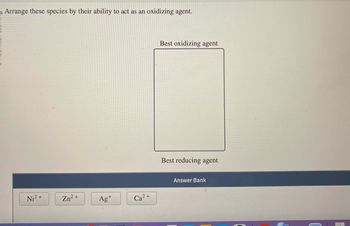
Transcribed Image Text:Arrange these species by their ability to act as an oxidizing agent.
Ni²+
Zn²+
Ag+
2+
Ca
Best oxidizing agent
Best reducing agent
Answer Bank
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (Incorrect) Consider the following redox reaction: Bi(OH)3(s) + Sn(OH)3(aq) → Sn(OH)²-(aq) + Bi(s) - (basic) What is the coefficient of Sn(OH)3 when the equation is balanced using the smallest whole number coefficients? 6 (Your answer) 5 2 3 (Correct answer) 1arrow_forwardPlease include the net ionic equations as well thanksarrow_forwardMacmillan Le For each reaction, identify the substance that is the oxidizing agent and the substance that is the reducing agent. C₂H4 Fe₂O3 2 KI E + + ****** H₂ *E***HELINES THEERTE E CO OTHEATERSHE *****INE DADES DE Cl₂ SER WEDNES. II C₂H6 2 FeO + CO₂ → 2 KCI + + 1₂ presented by Macmillan Learning Answer Bank reducing agent oxidizing agentarrow_forwardArrange these species by their ability to act as an oxidizing agent. Best oxidizing agent Poorest oxidizing agent Answer Bank Au3+ Mg2+ Cr2+arrow_forwardFor each reaction, identify the atom oxidized, the atom reduced, the oxidizing agent, and the reducing agent. 1) Mg + 2HCI a MgCl, + H, 2) 2Fe + 3V,0, Fe,O, +6VO 3) 2KMNO, + 5KNO, + 3H,SO, 2MnSO, + 3H,0 + 5KNO, +K,SO, 4) K,Cr,O, + 3SnCl, + 14HCI a 2C:CI, + 3SnCl, +2KCI + 7H,O 5) 2KMNO, + 10NaCl + 8H,SO, a 5CI, + K,SO, + 2MnSO, + 8H,O+ 5Na,SO, 6) 2K,Cr,O, + 2H,0+3S a 3s0, +4KOH+2Cr,O, 7) 8KCIO, + C,„H„O" 8KCI + 11H,0 +12CO, 8) 3HĻC,O, + 2K,MnO,6CO, + 2K,O+Mn,O, + 3H,O 9) 2Mn(NO,), + 5NaBiO, + 16HNO, 2HMNO, +5Bi(NO,), + 5NANO, + 7H̟O 10) 4H,C,O, + 2KMNO, 8CO, + K,O + Mn,O, + 4HĻOarrow_forwardSelect True or False: Under acidic conditions, the correctly balanced redox reaction forClO2-(aq) → ClO2(g) + Cl-(aq) is:5ClO2-(aq) + 4H+(aq) → 4ClO2(g) + Cl-(aq) + 2H2O(l) Group of answer choices True Falsearrow_forwardIdentify the oxidizing agent and reducing agent in the reaction.Te0 3 2- + 2 N204 + H20 -> Te + 4 NO3 - + 2H+arrow_forwardCu 2+ (aq) Zn 2+ + 34. Zn (s) + Cu (s) Identify which half reaction is oxidation and which is reduction. 2e Zn 2+ Zn (s) Cu 2+ (aq) Bed 2e (aq) Cu (s) 35. Write the equilibrium expression (Keq) for the following reversible reaction: N2(g) + O2(g) 2 NO (g) 36. For the reaction in question 35, if we add more N₂, which direction will the equilibrium a. To the right (forward) b. To the left (reverse) c. It won't make a differencearrow_forwardBalance the following oxidation-reduction occurring in acidic solution. MnO 4 ^ - (aq)+Co^ 2+ (aq) Mn^ 2+ (aq)+Co^ 3+ (aq) Identify the oxidation half-reaction, reduction half-reactionoxidizing agent and reducing agent as well as balancing in acidic conditions.arrow_forwardPredicting whether simple electrochemical reactions happen Decide whether a chemical reaction happens in either of the following situations. If a reaction does happen, write the chemical equation for it. Be sure your chemical equation is balanced and has physical state symbols. situation A strip of solid silver chemical reaction? metal is put into a beaker of 0.079M Fe(NO3)2 solution. yes no A strip of solid iron metal is put into a beaker of 0.084M AgNO3 solution. chemical equation ☐ yes ☐ no ☑arrow_forwardFor each reaction, identify the substance that is the oxidizing agent and the substance that is the reducing agent. C,H4 H, C,H, Answer Bank Fe,O3 CO 2 FeO + CO, + oxidizing agent reducing agent 2 KI Cl, 2 KCl + 12 +arrow_forward25arrow_forwardarrow_back_iosarrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY