
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
17a
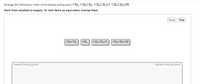
Transcribed Image Text:Arrange the following in order of increasing boiling point: CH4, CH3CH3, CH3CH2CI, CH3CH2OH.
Rank from smallest to largest. To rank items as equivalent, overlap them.
Reset Help
CH3 CH3
CH,
HOʻHƆ®HƆ
lowest boiling point
highest boiling point
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In an attempt to measure the effects of acid rain, researchers measured the pH (7 is neutral and values below 7 are acidic) of water collected from rain in Gurabo, Puerto Rico. 5.40 5.37 5.38 4.63 5.39 3.74 3.71 4.96 4.64 5.12 5.65 5.36 4.16 5.87 4.57 4.62 5.48 4.57 4.57 4.53 4.82 4.52 4.69 4.32 3.98 5.70 4.35 3.98 5.68 3.44 5.04 4.67 4.51 4.34 4.16 4.64 5.12 3.74 4.64 5.59 Draw the correct histogram. Many scientists consider rain with pH below 5.3 to be acid rain. What percentage of these samples could be considered as acid rain? Note: You may MS Excel, but you must submit the filearrow_forwardPh and pOh of 0.020m HNO3arrow_forwardCalculate the pH of a 0.123 M solution of ethylenediamine (H₂NCH₂CH₂NH₂). The pKą values for the acidic form of ethylenediamine (H3NCH₂CH₂NH³) are 6.848 (pKal) and 9.928 (pKa2). pH = Calculate the concentration of each form of ethylenediamine in this solution at equilibrium. [H₂NCH₂CH₂NH₂] : = [H₂NCH₂CH₂NH3] = M Marrow_forward
- All of the following statements about the pH scale are true EXCEPT O Cola drinks are very acidic. O The difference in concentration between pH10 and pH 12 is 20. O Neutral is pH 7.00 O Blood is slightly basic. O pH 2 is a strong acid.arrow_forwardIdentify the TRUE statement about acid rain. O Acid rain dissolves marble statues. O Acid rain contains nitric acid and sulfurous acid O Acid rain comes from hydroelectric power plants. O NO combines with oxygen and water to form nitrous acid, which is a component of acid rain. O Acid rain does not corrode steel bridges.arrow_forwardWrite the hydrolysis reaction for the hypochlorite ion, ClO-.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY