
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
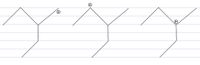
Arrange from most to least stable
Expert Solution
arrow_forward
Step 1
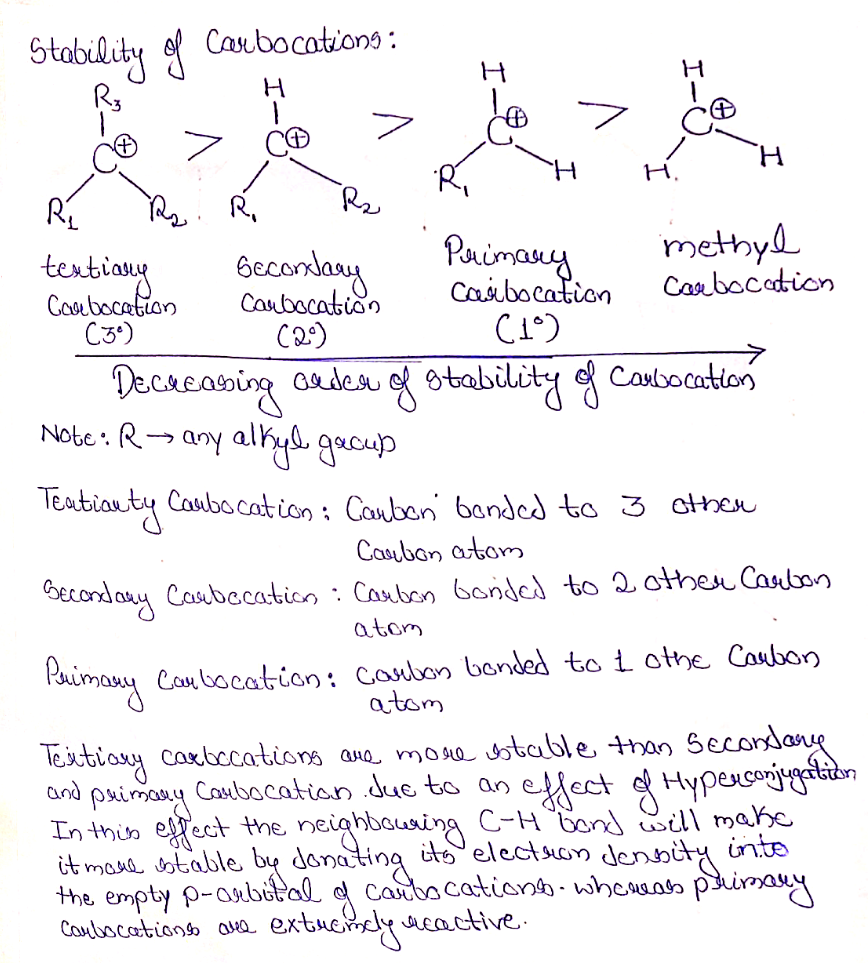
Stability of carbocation:
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Mono addition of HBr to conjugated dienes leads to multiple products, and a given addition product can arise from more than one conjugated diene. Two allylic bromide products can be synthesized from a diene. From the list given, identify the pair of dienes wherein each diene can serve as a precursor for both of the addition products. Note that not all possible products are shown. Diene HBr Identify the diene precursors. H3C مک می H3C. Br CH3 + Br H3C Two monoaddition products CH3 CH3arrow_forwardsystematic (IUPAC) name: H3C-CH2-NH2-H3C-CH2-CH-CH2 I CH3 NH2 I CH-CH3 H3C-CH2-N-CH2-CH3 I H NH-CH3 I H3C-CH-CH3 H3C-N-CH3 I H2C-CH2 -CH-CH3 H3C-N-CH2-CH3-H3C-CH2-CH2-CH2arrow_forward10-5. Does BaSO, or KCN have the lower melting point?arrow_forward
- true or false O2-(g) is more stable than O-(g).arrow_forwardSelect the best name for the given compound. H. Select the name. O trans-bicyclo[3.2]heptane O trans-bicyclo[3.2.0]heptane O trans-bicyclo[0.2.3]heptane cis-bicyclo[3.2.0]heptane cis-bicyclo[3.2]heptane O cis-bicyclo[0.2.3]heptanearrow_forwardGiven: C₂H4(g) +302(g)+2CO₂(g)+2H₂O(g) AH(C₂H4)= 52.5 kJ/mol AH (H₂O)=-285.8 kJ/mol A Hºrxn=-1411.1 kJ/mol. Find AH₁(CO₂)=. View Available Hint(s) O-1358 kJ/mol O +393.5 kJ/mol O-1303.5 kJ/mol O-393.5 kJ/mol Submit harrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY