
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
In part (i) write a BALANCED equation and in part (ii) answer the questions about the reaction. In part (i), coefficients should be in terms of lowest whole numbers. Assume that solutions are aqueous unless otherwise indicated. Represent substances in solutions as ions if the substances are extensively ionized. Omit formulas for any ions or molecules that are unchanged by the reaction.
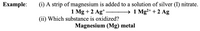
Transcribed Image Text:Еxample:
(i) A strip of magnesium is added to a solution of silver (I) nitrate.
1 Mg + 2 Ag*
(ii) Which substance is oxidized?
Magnesium (Mg) metal
→ 1 Mg²+ + 2 Ag

Transcribed Image Text:Aqueous hydrobromic acid is added to a beaker How could the aqueous product here be distinguished
from the aqueous product formed if strontium
containing solid calcium carbonate.
carbonate were substituted for calcium carbonate?
i) Balanced equation:
ii) Answer:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please don't provide handwritten solution .....arrow_forward81. For each of the following pairs of formulas, predict whether the substancesthey represent would react to yield a precipitate. (The products formed in the reactions that take place are used as a catalyst, as a tanning agent, as a pigment, in fertilizers, as a food additive, and on photographic film.) If there is no reaction, write, “No Reaction”. If there is a reaction, write the complete equation for the reaction. a. KOH(aq) + Cr(NO3)3(aq)b. Fe(NO3)3(aq) + K3PO4(aq)c. NaBr(aq) + AgNO3(aq)d. Mg(C2H3O2)2(aq) + NaCl(aq)arrow_forward3. Write the balanced complete molecular, complete ionic and net ionic chemical equations including phase labels for the neutralization of solid potassium hydroxide with aqueous sulfuric acid. Include phase labels.arrow_forward
- 3. A solution contains silver nitrate, lead (II) nitrate, and calcium nitrate. What reagent could be added to this solution that would separate the calcium nitrate from the other three compounds? Write the formula of the resulting precipitates after the addition of this reagent.arrow_forwardWhen each of the following pairs of aqueous solutions is mixed, does a precipitation reaction occur? If so, write the formula and name of the precipitate. (Type your answer using the format CO2 for CO2. Type NONE in the blanks if there is no precipitation reaction.) (a) lead(II) nitrate + potassium bromideformulaname(b) sodium sulfide + nickel(II) sulfateformulanamearrow_forward8)Does a reaction occur when aqueous solutions of potassium hydroxide and sodium nitrate are combined? yes or no If a reaction does occur, write the net ionic equation.arrow_forward
- 1) Write the type of reaction. 2) Write the chemical equation. Do not worry about balancing this reaction. Just be sure that you have written the correct formulas and states. solid iron and aqueous copper(II) bromide react to form solid copper and aqueous iron(II) bromide.arrow_forwardWrite the balanced equation that occurs when aqueous sodium carbonate reacts with aqueous iron (III) chloridearrow_forwardMagnesium oxide is sometimes used as an antacid to help with indigestion and stomach upset caused by excess stomach acid (hydrochloric acid). Write two balanced chemical equations to explain how this works. One should be a synthesis and the other a neutralization.arrow_forward
- 14.4 g of magnesium nitrate is dissolved in 1.20 L of water. 25 mL of this solution is taken and diluted to 175 mL with water. What is the final concentration of the nitrate ion?arrow_forwardAll ionic compounds dissolve in water. True Falsearrow_forwardA precipitation reaction involves the formation of a precipitate when aqueous solutions are mixed. Not all combinations of aqueous solutions produce precipitates, and it is important to be able to predict the ones that do. A) Complete and balance the molecular equation for the reaction between aqueous solutions of lithium fluoride and potassium chloride, and use the states of matter to show if a precipitate forms. B) Write the complete ionic equation for the reaction that takes place when aqueous solutions of lithium fluoride and potassium chloride are mixed. C) Write the net ionic equation for the precipitation reaction, if any, that may occur when aqueous solutions of lithium fluoride and potassium chloride are mixed. If there is no net ionic equation, simply write "none."arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY