
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
Transcribed Image Text:Hew folder
Genetic drift - Wikip.
Algebra Foundation.
s Lab
Add-ons Help
Last edit was yesterday at 10:48 AM
BIUA
Russo One
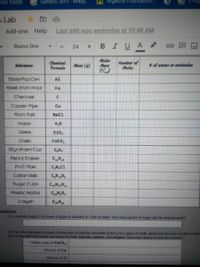
24
Chemieal
Formida
Molar
Mart
Number of
Moles
Subetance
Mass (9)
of atoms or molecules
Soda Pop Can
Al
Steel lironl Wool
Fe
Charcoal
Copper Ppe
Cu
Rock Salt
Nacl
Water
Glass
Si0,
CaCo,
Styrofoam Cup
C,H
Pencil Eraser
C,H,
PVC Pipe
C,H,Cl
Cotton Ball
C,H 0,
Sugar Cube
CH0
Plasbc Bottle
Crayon
uestions
(1) A chemist needs 1.25 moles of sugar to dissolve in 1 iter of water. How many grams of sugar will the chemist need?
(2) From the calculaled number of molecules of calcium carbonate (Caco) in a piece of chalk, determine the lotal number of alon
each at the elements within vour piece of chalk calcium, carbon, and oxygen. How many atoms in total are present?
Molecules of CaCO,
Roms of Ca
Ftoms of C
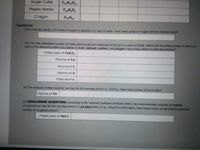
Transcribed Image Text:Sugar Cube
CH0
Plastic Bottle
CH,0
Crayon
CasHa
Questions
(1) A chemist needs 1.25 moles of sugar to dssolve in 1 iter of water. How many grams of sugar will the chemist need?
(2) From the calculated number of molecules of calcium carbonate (Cac0,) in a piece of chalk, determine the total number of atoms of
each of the elements within your piece ot chalk calcium, carbon, and oxygen. How many atoms in total are present?
Molecules of CaCo,
Atoms of Ca
Atoms of C
Atoms of 0
Total atomS
(3) The amount of iron required per day for the average person is D.015 g. How many atoms of ron is this?
Atoms of Fe:
(4) CHALLENGE QUESTION: According to the National Insututes oT Health (NH), the recommended amount of sodium
consumed per day for the average person is no more than 2.3 g. Using this information, how many moles of salt (NaC) would be
healthy for a typical person?
Molecules of Nacl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ten billion molecules of water is decomposed into hydrogen and oxygen. How many molecules of hydrogen gas and oxygen gas are formed?arrow_forwardAssume that the buret below was previously filled to the 0.00 mL mark. What volume of reagent has been delivered? (Remember to estimate between the lines to get one more digit than can be read from the scale and include units!) 1. 2.arrow_forwardWould a car be able to travel more miles on a gallon of gasoline or a gallon of gasohol?arrow_forward
- Determine the molar mass of Cu(NO,),. Provide an answer to two decimal places.arrow_forwardThe mole is a unit that counts things and it can be converted into [Select all that are correct.] mass (g) volume of gas a number of particlesarrow_forwardA residue of potassium chloride will be left in the "container" after the heating is completed. Do you expect it weigh more than, less than or the same as the original potassium chlorate sample? Why?arrow_forward
- Buried Treasure: If they mine 100 tons of copper-ore, how much copper is actually gained?arrow_forward5:02 Question 4 of 12 Submit Gasoline is composed of a variety of different liquid hydrocarbons, which do not separate as time passes. Gasoline is an example of a: A) heterogeneous mixture B) Chemical compound C) Chemical element D) Solutionarrow_forwardWhat is the mass, in grams, of 28.62 mLmL of acetone?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY