
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
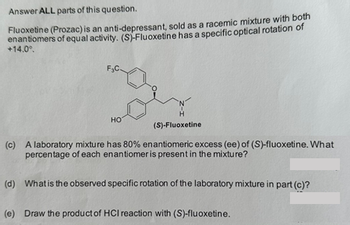
Transcribed Image Text:Answer ALL parts of this question.
Fluoxetine (Prozac) is an anti-depressant, sold as a racemic mixture with both
enantiomers of equal activity. (S)-Fluoxetine has a specific optical rotation of
+14.0°.
F₂C.
HO
(S)-Fluoxetine
(c) A laboratory mixture has 80% enantiomeric excess (ee) of (S)-fluoxetine. What
percentage of each enantiomer is present in the mixture?
(d) What is the observed specific rotation of the laboratory mixture in part (c)?
(e) Draw the product of HCI reaction with (S)-fluoxetine.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following statements is/are true of compound C given below? OH HO Compound C OH (i) The boiling point of compound C is higher than the boiling point of (ii) The chirality centre (stereocentre) of compound C is of S configuration. CI (iii) Compound C reacts with SOCI, in pyridine to form CI O Only statements (ii) and (iii) are true. O All three statements are true. O Only statement (i) is true. O Only statement (ii) is true. O Only statement (iii) is true.arrow_forwardAssign R or S configurations to each stereogenic center of isoborneol and borneol. Give the relationship between these two stereoisomers.arrow_forwardOne commercial synthesis of flurbiprofen (the active ingredient in Ansaid and a score of other over-the-counter and prescription nonsteroidal anti-inflammatory drug preparations) gives the enantiomer shown in 94% enantiomeric excess. H CH3 он (a) Assign an R or S configuration to this enantiomer of flurbiprofen. OR What are the percentages of R and S enantiomers in the mixture? (Enter unrounded values.) (b) R: % S: 96arrow_forward
- Why does the melting point (70-71) above match neither the enantiomers’ nor the racemate’s? Does it mean that the substance is impure, or perhaps isn’t even ibuprofen at all? (Racemic mixture:75-77C, Enantiomer:52-53C)arrow_forwardPlease help me how to solve this problem in detail so i can understand it. Thank you very much.arrow_forwardplease answer all parts , thank you :)arrow_forward
- Which is/are optically inactive? CH2CH3 HO- H- H- CH3 H OH H- H. CH3 ОН, H- OH но H. CH3 CH3 CH3 I II III I and II Il only IIl only Il and III A. В. С. D. O D A O Barrow_forwardName the compounds shown below. Use an R of an S to label each chirality centerarrow_forwardQ8. Draw the two possible chair-like conformations of compound 1. 2 Using the Cahn Ingold Prelog priority rules, assign the absolute stereochemistry to each stereogenic centre contained in compound 1. NH2 1arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY