
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
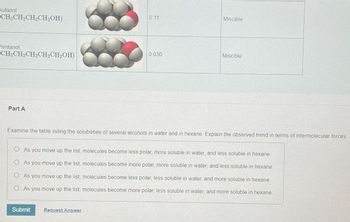
Transcribed Image Text:Butanol
CH3CH₂CH₂CH₂OH)
Pentanol
CH3CH₂CH₂CH₂CH₂OH)
Part A
0.11
Submit
0.030
Request Answer
Miscible
Examine the table listing the solubilities of several alcohols in water and in hexane. Explain the observed trend in terms of intermolecular forces.
Miscible
OAs you move up the list, molecules become less polar, more soluble in water, and less soluble in hexane.
O As you move up the list, molecules become more polar, more soluble in water, and less soluble in hexane.
As you move up the list, molecules become less polar, less soluble in water, and more soluble in hexane.
O As you move up the list, molecules become more polar, less soluble in water, and more soluble in hexane
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- m A 50.0-g sample of ethyl alcohol (C2H5OH) is dissolved in 75.0 of water. What is the mole fraction of ethyl alcohol? 80gFth + 200g H₂0 na 0.207 0.342 TOS mol 0.414 0.667arrow_forwardChoose the statement below that is true. A solution will form between two substances if the solute-solvent interactions are of comparable strength to the solute-solute and solvent-solvent interactions. A solution will form between two substances if the solute-solvent interactions are small enough to be overcome by the solute-solute and solvent-solvent interactions. A solution will form between two substances if the solute-solute interactions are strong enough to overcome the solvent-solvent interactions. A solution will form between two substances only if the solvent-solvent interactions are weak enough to overcome the solute-solvent interactions. None of the above.arrow_forwardplease help asap (Be sure your answer has the correct number of significant digits.)arrow_forward
- Estimate the composition of N2 dissolved in water at 298 K. The partial pressure of N2 at this temperature is 44.5 atm. The Henry's law constant for N2 in water is HN2-H2O=87008.55 atm xN2 HN2-H2O= yN2 P= PN2=44.5 atm xN2=44.5 atm/87008.55 atm Legible lettering and step-by-step, please.arrow_forwardSolids are more soluble at high temperatures and gases are less soluble at higher temperatures. True Falsearrow_forward6. two liquids that UNIFORMLY mix when combined are described as Group of answer choices polar immiscible miscible heterogeneousarrow_forward
- Use the 'like dissolves like' to predict weather each of the following should be soluble in water. a- benzene, C6H6 b- ethylene glycol, HOCH2CH2OH c- potassium iodine, KIarrow_forwardDetermine if each sold can completely dissolve in a given amount of water at the indicated temperature.arrow_forward* 00 LL LI #3 The normal freezing point of a certain liquid X is -2.70 °C, but when 43.3 g of potassium bromide (KBr) are dissolved in 400. g of X the solution freezes at -9.6 °C instead. Use this information to calculate the molal freezing point depression constant K, of X. Round your answer to 2 significant digits. °C-kg K. mol Check Save For Later Submit Assignme O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Accessibil MacBook Pro 114 F8 F3 F4 $4 3. 5. 6. P. A G H. K. B alt alt option command command optionarrow_forward
- Pleaseeeeeeeeeeeeearrow_forwardThe normal boiling point of a certain liquid X is 131.10°C, but when 32.9g of glycine (C2H5NO2) are dissolved in 600.g of X the solution boils at 132.1°C instead. Use this information to calculate the molal boiling point elevation constant Kb of X.Round your answer to 2 significant digits. Please do not round untill the end =Kb⋅°Ckgmolarrow_forwardThe normal temperature range of the liquid phase of pure water is 0°C to 100°C. Which of the following solutions will have the largest temperature range for the liquid state? a. 0.10 M sodium phosphate b. 0.10 M sodium chloride c. 0.10 M sodium sulfate d. 0.10 M sodium carbonatearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY