
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A neutral solution of water at a particular temperature has a concentration of OH- of 4.5 × 10-7 M. What is Kw at this temperature?
Expert Solution
arrow_forward
Step 1
Water molecule can act as acid as well as base. Hence it can give or take H+ ions. The auto ionization of water can be written as:
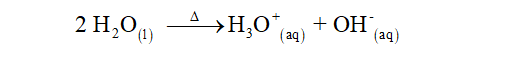
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- when 3 L of silver nitrate and 3 L of hydrochloric acid, both at 0.25 M, are mixed, a precipitate is formed. The enthalpy change for the reaction is -45 J. Calculate the enthalpy change in kJ/mol of silver chloride.arrow_forwardQUESTION 10 (4b-401-1.53-1) An intern working in a research lab knocks over a beaker containing an important solution. The beaker originally contained 1.50 L of a 1.53 M solution, but after it spilled it only contained 1 L of solution. The intern decides to hide his mistake by refilling the beaker to contain 1.50 L again by adding pure water (not realizing that this will dilute the solution). What is the new concentration of the solution? Show all work and give your answer with three sig figs. Click Save and Submit to save and submit. Click Save All Answers to save all answers. Save All Answer 1600 4,104 PAGES APR O P W MacBook Air DII DD 80 000 000 F7 F8 F9 F10 F2 F3 F4 F5 F6 F1 #3 $ & 2 3 4 6 7 8. W E T Y P R ... 2.arrow_forwardSodium hydrogen carbonate NaHCO3, also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid HCl, which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: HCl(aq)+NaHCO3(aq)→NaCl(aq)+H2O(l)+CO2(g) The CO2 gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 200.mL of a 0.089M HCl solution. What mass of NaHCO3 would she need to ingest to neutralize this much HCl? Be sure your answer has the correct number of significant digits.arrow_forward
- [References] A sample of an industrial waste water is analyzed and found to contain 48.5 ppb Co³+. How many grams of colbalt could be removed from 1.98×10³ kg of this waste water? grams Coarrow_forwardThe solubility of AgBrO, in water at 25 °C is measured to be 1.7 Use this information to calculate K for AgBrO3. sp Round your answer to 2 significant digits. x10arrow_forwardA salt has a solubility in water of 2.5 g/L at 25 °C Suppose you mixed 10.0g of this salt with 3.0 L of water in a beaker and allowed the salt to dissolve. The temperature of the solution was kept at 25 °C. Would there be any solid left in the beaker after the salt has dissolved? Select one: O Yes, there would be some solid would be left in the beaker. O No, there would not be any solid left in the beaker. Not enough information is provided to answer this questionarrow_forward
- 7B.arrow_forwardSuppose you want to measure the amount of glucose in a can of soda. You can use the data generated in this experiment to help you, but what other data would you need to collect in order to measure the concentration of glucose in the soda? In other words, what additional experimental step(s) would you perform? B I U Ꭶ X X, 14▾ A In order to measure the amount of glucose in a can of soda, one would need to prepare a series of standard solutions w/ an unknown concentrations of glucose using the 0.3M of glucose sol'n and DI water. Therefore, the glucose oxidase assay would be performed on each standard sol'n and the result values would also be measured by a spectrophotometer. A standard curve may then be plotted by absorbances and the unknown concentration of glucose. It may also be necessary to adjust the pH of the soda can sample to preform additional steps. Change Submission Submitted. Waiting on graded response. 14) Once you have collected data from the step(s) proposed in the previous…arrow_forwardThe reaction A(aq) + B(aq) C(aq) + D(aq) has a ΔHrxn = -85 kJ/mol. This reaction is endorthermic or exothermic . If the temperature of the reaction mixture is increased, equilibrium will shift towards the reactans or products. This change will decrease or increase the concentration of the reactants and K will increase or deacreasearrow_forward
- 100 90 80 NANO, 70 60 CaCl 50 Pb(NO.)2 40 NaCi KCI 30 20 KCIO, 10 Ce,(SO O 10 20 30 40 50 60 70 80 90 100 Temperature ("C) How many grams are needed to create a saturated solution of Pb(NO3)2 at 30°C if you have 20 grams of Pb(NO3), already dissolved in a solution? Solubility (g of salt in 100 g H,O) SONYarrow_forwardWhat is the minimum volume of 2.47 mol L−1 HCl(aq) required to dissolve 12.3 g Mn metal? The atomic weight of Mn is 54.94 g mol−1. Mn(s) + HCl(aq) ⟶ MnCl2(aq) + H2(g)arrow_forwardDetermine if the reaction favors the reactants or the products.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY