
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%

Transcribed Image Text:Procedure:
Measurements and Observations:
0.88
1. Place a weigh boat on the top-loading
balance and press the TARE button.
Add one almond and record the mass
with the correct units and to the correct
number of significant figures.
mass of 1 almond =
2. Return the almond to the bin container.
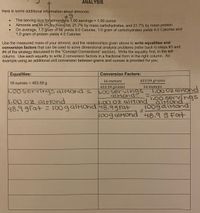
Transcribed Image Text:ANALYSIS
Here is some additional information about almonds:
The serving size for almonds is 1.00 servings 1.00 ounce
Almonds are48.9% by mass fat, 21.7% by mass carbohydrates, and 21.7% by mass protein
On average, 1.0 gram of fat yields 9.0 Calories, 1.0 gram of carbohydrates yields 4.0 Calories and
1.0 gram of protein yields 4.0 Calories
Use the measured mass of your almond, and the relationships given above to write equalities and
conversion factors that can be used to solve dimensional analysis problems (refer back to steps #3 and
#4 of the strategy discussed in the "Concept Connections" section). Write the equality first, in the left
column. Use each equality to write 2 conversion factors in a fractional form in the right column. An
example using an additional unit conversion between grams and ounces is provided for you.
Equalities:
Conversion Factors:
16 ounceS
453.59 grams
16 ounces = 453.59 g
453.59 grams
16 оипсes
T.00 0z alMOnd
.00 servirngs
alMond
100gaiMond
1.00servings alMond
1.00 servings
alMonds
1.00 0Z alMond
48.9 9Fat = 100galMond 48.99Fat
1.00 0z alMond
%3D
100g alMond 48.9 9 Fat
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the reaction given below, how much energy is produced during the reaction of 72.5 g Cl₂ and 2.5 g B2H6 ? B2H6(g) + 6 C12(g) →2 BC13(g) + 6 HCl(g) 3070 kJ 1640 kJ 126 kJ 1430 kJ 429 kJ AH*rxn = -1396 kJarrow_forward12arrow_forwardMass in one serving (g) Calories in one serving (kcal) Fat 8 Carbohydrates 37 Protein 3 Calculated total Calories per-serving(kcal): Total Calories per serving from label: 230 Calarrow_forward
- 1 ! QUESTION 10 The metabolism of one glucose with six oxygens to form six carbon dioxides and six waters releases 678 kcal per mole of glucose. Given this, which statement below is true? O Glucose metabolism is endothermic and the energy of the products (CO2 and H2O) are lower than the reactants (glucose and O2) O Glucose metabolism is endothermic and the energy of the products (CO2 and H₂O) are higher than the reactants (glucose and O2) O Glucose metabolism is exothermic and the energy of the products (CO2 and H2O) are higher than the reactants (glucose and O2) O Glucose metabolism is exothermic and the energy of the products (CO2 and H2O) are lower than the reactants (glucose and MacBook Pro C G Search or type URL A Q A K 2 W S #3 E D $ 4 R F do LO % 5 T 6 G Y & 7 H ☆ U * 00 8 9 O J Karrow_forwardWould the following reaction be endothermic or exothermic? Why? CH4 + O2 --> CO2 + H2O + Energy O Endothermic because energy is a product Endothermic because energy is a reactant O Exothermic because energy is a reactant O Exothermic because energy is a productarrow_forwardBoth animals and plants rely on stored energy for survivalAnimals can store energy in the form of sugars from the consumption of other matter. Plants the other hand, utilize photosynthesis to produce sugar (glucose). Following storage, the sugars can then be used to provide energy for various functions within the organism. This is referred to as the burning of sugar shown in the chemical reaction below: C 6 H 12 O 6 (s)+O 2 (g) CO 2 (g)+H 2 O(g) 3. Complete the following questions based on the reaction above representing burning sugar: . Balance the chemical equation representing sugar burning (reaction above). b. What type of reaction(s) is/are being represented by the chemical reaction in part (a) ? . How would you prepare 40.00 of 0.250 M sugar solution (C 6 H 12 O 6 ) from a stock solution that is 1.25 M C 6 H 12 O 6 (aq)? ? Provide your answer in terms of of sugar stock solution and explain how you would dilute to the total volume. (HintHow many of sugar stock solution and how…arrow_forward
- Consider these reactions: Reaction 1: H₂(g) + Cl₂ (g) 2HCl(g) AH-184.6 kJ Reaction 2: 20F2(g) O₂(g) + 2 F₂ (g) AH = -49.4 kJ Reaction 3: N₂(g) + 2O₂(g) · 2NO₂(g) AH = +66.4 kJarrow_forwardheat content in the surroundings of an endothermic reaction decreases, because the energy contained in the products is lower than the reactants the reaction absorbs heat energy the energy contained in the reactants is lower then the products heat energy is destroyed during reactionsarrow_forwardDetermine Kc for the reaction 1 1 1 N₂(8) + 0₂ (8) + Br₂ (8) = NOBr(g) 2 2 based on the following information 2NO(g) = N₂(g) + 0₂ (8) NO(g) + Br₂(g) = NOBr(g) K=2.1x1030 C K = 1.4 Carrow_forward
- Label both formulas shown in both reactions as anhydrates and hydrates.arrow_forward21. Which device to nutritionists use to determine the number of calories in samples of food? a foam cup filled with water a bomb calorimeter a spectrometer a constant pressure calorimeterarrow_forward23. Salt A and salt B were dissolved separately in 100-milliliter beakers of water. The water temperatures were méasured and recorded as shown in the table below: Salt A Salt B Initial water temperature: 25.1°C Final water temperature: 25.1°C 20.0°C 30.2°C Which statement is a correct interpretation of these data? a) The dissolving of only salt A was endothermic b) The dissolving of only salt B was exothermic c) The dissolving of both salt A and salt B was endothermic đ) The dissolving of salt A was exothermic and the dissolving of salt B was endothermic 24. The graph to the right represents the uniform cooling of a substance, starting with the substance as a gas above its boiling point. How much time passes between the first appearance of the liquid phase of the substance and the presence of the substance completely in its solid phase? a) 5 minutes b) 2 minutes c) 7 minutes d) 4 minutes e) impossible to tell from the information given 150- 100- 50- 01+23 4 5 6 7 8 9 10 11 12 13 14 25.…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY