
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
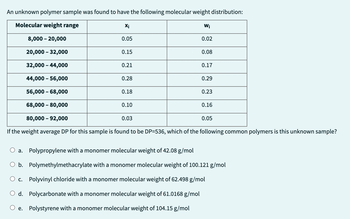
Transcribed Image Text:An unknown polymer sample was found to have the following molecular weight distribution:
Molecular weight range
8,000 - 20,000
20,000 - 32,000
32,000 - 44,000
44,000 - 56,000
56,000 - 68,000
68,000 - 80,000
80,000 - 92,000
If the weight average DP for this sample is found to be DP=536, which of the following common polymers is this unknown sample?
a.
Xi
e.
0.05
0.15
0.21
0.28
0.18
0.10
0.03
Wi
0.02
0.08
0.17
0.29
0.23
0.16
Polypropylene with a monomer molecular weight of 42.08 g/mol
O b. Polymethylmethacrylate with a monomer molecular weight of 100.121 g/mol
c.
Polyvinyl chloride with a monomer molecular weight of 62.498 g/mol
d.
Polycarbonate with a monomer molecular weight of 61.0168 g/mol
Polystyrene with a monomer molecular weight of 104.15 g/mol
0.05
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- QUESTION 26 A pleasant-smelling liquid having a boiling point of 101°C was subjected to IR Spectroscopy to determine the functional groups present and partially determine its nomenclature based on the molecular formula given. What are the functional groups present based on the major infrared absorptions? (Select Possible Answers) 100 CH1202 '2876 2977 1194 liquid film sample 1737 1166 4000 3000 2000 1500 1000 500 wavenumber cm Ester Alkene Alkane Alkyne Alcohol Carboxylic Acid Ketone Aldehyde Transmittance %Tarrow_forwardWhich of the statements below apply to the following reaction: -OH + HNR' H -NR' + H20 H R R Choose all that apply. Select at least one answer to see feedback. Da condensation reaction an esterification reaction the formation of an amide linkage the formation of an ether linkage an addition reactionarrow_forwardHow would you do number 8 a-farrow_forward
- After looking the chemical structure of nylon (https://en.wikipedia.org/wiki/Nylon) what interaction would cause a dye to bind to nylon fabric? dipole-dipole electrostatic hydrogen bonding van der waals/london dispersionarrow_forwardhelp please answer in text form with proper workings and explanation for each and every part and steps with concept and introduction no AI no copy paste remember answer must be in proper format with all workingarrow_forward4 uv/vis spectrum obtained by my TAarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY