
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
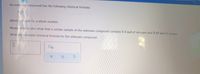
Transcribed Image Text:An unknown compound has the following chemical formula:
NO
where stands for a whole number,
Measurements also show that a certain sample of the unknown compound contains 8.4 mol of nitrogen and 8.43 mol of oxygen.
Write the complete chemical formula for the unknown compound.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 6. This Magnesium top by Foreverspin TM weighs merely 6g. a) Write the weight of this top in kilograms, in scientific notation. b) The Atomic Weight of Magnesium is 24.305 grams/ Mole. If there are 6.02214076 ×x 1023 atomic particles in 1 Mole of Magnesium, how many particles are in 6g? Operate and express your solution in scientific notation and in a sentence.arrow_forwardWrite the complete chemical formula for the unknown compound.arrow_forwardWhat is a mole? How is the mole concept useful in chemical calculations?arrow_forward
- An unknown compound has the following chemical formula: N,O3 where x stands for a whole number. Measurements also show that a certain sample of the unknown compound contains 9.1 mol of nitrogen and 13.45 mol of oxygen. Write the complete chemical formula for the unknown compound.arrow_forwardIn a combustion reaction, 31.0 g of hydrocarbon reacts with 96.0 g of oxygen to produce water and carbon dioxide. If 63.0 g of water is produced, how much carbon dioxide is produced?arrow_forwardAn unknown compound has the following chemical formula: P,S where x stands for a whole number. Measurements also show that a certain sample of the unknown compound contains 7.2 mol of sulfur and 2.87 mol of phosphorus. Write the complete chemical formula for the unknown compound. P3S5arrow_forward
- An unknown compound has the following chemical formula: NO, where X stands for a whole number. Measurements also show that a certain sample of the unknown compound contains 7.8 mol of nitrogen and 15.00 mol of oxygen. Write the complete chemical formula for the unknown compound.arrow_forwardBe sure to answer all parts. Calculate the number of atoms in 0.422 g of potassium (K). Enter your answer in scientific notation. x 10 atomsarrow_forwardHow many moles of iron atoms do you have if you have if you have 8.00 × 1023 atoms of iron. ( The mass of one mole of iron is 55.85g.)arrow_forward
- Assume you have 38.0 grams of CO (carbon monoxide), which is a molecular compound. How many molecules of CO are present?arrow_forwardHow many grams of rubidium are in 3.49 x 1024 atoms of rubidium? Enter your answer in decimal form with the correct number of sig figs. Use the proper abbreviation for the units.arrow_forwardYou are given 1.0g samples of Iron (Fe), Silicon (Si), and Lithium (Li). Which of the three samples will contain the largest number of atoms? Which will contain the smallest number of atoms? In your answer, be sure to show your calculations OR your reasoning explaining youarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY