
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
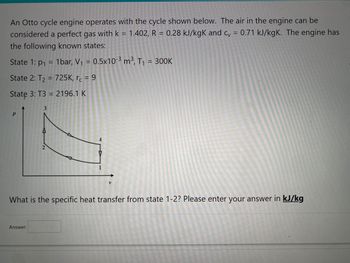
Transcribed Image Text:An Otto cycle engine operates with the cycle shown below. The air in the engine can be
considered a perfect gas with k = 1.402, R = 0.28 kJ/kgK and cv = 0.71 kJ/kgK. The engine has
the following known states:
State 1: p₁ = 1bar, V₁ = 0.5x10-³ m³, T₁ = 300K
State 2: T₂ = 725K, rc = 9
State 3: T3 = 2196.1 K
P
3
Answer:
2
V
What is the specific heat transfer from state 1-2? Please enter your answer in kJ/kg
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- An Otto cycle engine operates with the cycle shown below. The air in the engine can be considered a perfect gas with k = 1.402, R = 0.28 kJ/kgk and cv = 0.71 kJ/kgK. The engine has the following known states: State 1: p₁ = 1bar, V₁ = 0.5x10-³ m³, T₁ = 300K State 2: T₂ = 725K, rc = 9 State 3: T3 = 2196.1 K Р 3 Answer: 2 What is the specific internal energy at state 2? Please enter your answers in kJ/kg.arrow_forwardTwo ideal Brayton cycles (see Figure below) have the same inlet temperature and the same maximum temperature of the turbine inlet. Brayton Cycle A has a higher compression ratio than Cycle B. Which of the following statements is true about the cycles? Cycle A has a higher thermal efficiency and Cycle B has a higher work net for a given mass flow rate. Cycle B has a higher thermal efficiency and a higher work net for a given mass flow rate. Since the temperatures are the same the efficiencies and work net are also the same. Cycle A has a higher work net for a given mass flow rate and Cycle B has a higher thermal efficiency. Cycle A has a higher thermal efficiency and a higher work net for a given mass flow rate.arrow_forwardAn Otto cycle engine operates with the cycle shown below. The air in the engine can be considered a perfect gas with k = 1.402, R = 0.28 kJ/kgk and c, = 0.71 kJ/kgK. The engine has the following known states: State 1: p₁ = 1bar, V₁ = 0.5x10-3 m³, T₁ = 300K State 2: T₂ = 725K, rc = 9 State 3: T3 = 2196.1 K Р What is the specific heat transfer from state 3-4? Please enter your answer in kJ/kg Answer:arrow_forward
- An Otto cycle engine operates with the cycle shown below. The air in the engine can be considered a perfect gas with k = 1.402, R = 0.28 kJ/kgk and cv = 0.71 kJ/kgK. The engine has the following known states: State 1: p₁ = 1bar, V₁ = 0.5x10-3 m³, T₁ = 300K State 2: T₂ = 725K, rc = 9 State 3: T3 = 2196.1 K Р 3 Answer: 1 V What is the volume at state 2? Please enter your answers in m³.arrow_forwardAn Otto cycle engine operates with the cycle shown below. The air in the engine can be considered a perfect gas with k = 1.402, R = 0.28 kJ/kgk and cv = 0.71 kJ/kgK. The engine has the following known states: State 1: p₁ = 1 bar, V₁ = 0.5x10-3 m³, T₁ = 300K State 2: T₂ = 725K, rc = 9 State 3: T3 = 2196.1 K P 3 Answer: 2 4 What is the specific heat transfer from state 2 to 3? Please enter your answer in kJ/kgarrow_forward. Exercise 2 Compute the efficiency of the cycle shown in the figure below, under the assumption that the fluid used is an ideal diatomic gas. The A → B transformation is isothermal, the B →C transformation is isochoric and the C→ A transformation is adiabatic. The compression ratio VB/VA = 3. B Varrow_forward
- In a 4-stroke ideal diesel cycle; The cylinder volume is 0.96 liters when the piston is in bottom dead center, and the cylinder volume is 0.06 liters when it is in top dead center. At the beginning of compression, the temperature is T1 = 336 K, P1 = 98 kPa. 1.64 kJ / cycle heat is released as a result of combustion. The mechanical efficiency of the 6-cylinder engine operating at n = 1860 rpm is m = 0.84. Since Cv = 716.5 J / kg K, Cp = 1003.5 J / kg K, R = 287.13 J / kg K; a) Thermodynamic values at the cycle points, b) Effective power of the engine.arrow_forwardFor a Rankine cycle, increasing maximum temperature while holding all other parameters constant will allow an engineer to make the following statements Group of answer choices Quality at the turbine exit will decrease increasing liquid water content and causing turbine erosion. Quality at the turbine exit will increase and improve turbine durability. Increasing maximum temperature increases the average temperarture at which heat is added and will increase thermal efficiency Increasing maximum tempearture will decrease required mass flow rate for a specified power output.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY