
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
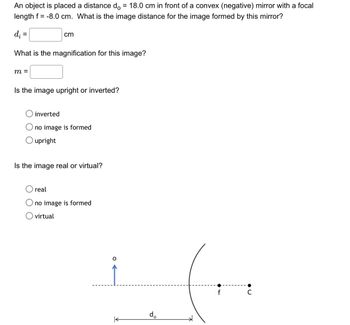
Transcribed Image Text:An object is placed a distance do = 18.0 cm in front of a convex (negative) mirror with a focal
length f = -8.0 cm. What is the image distance for the image formed by this mirror?
d₁ =
cm
What is the magnification for this image?
m =
Is the image upright or inverted?
inverted
no image is formed
O upright
Is the image real or virtual?
real
Ono image is formed
virtual
do
f
•
SAVE
AI-Generated Solution
info
AI-generated content may present inaccurate or offensive content that does not represent bartleby’s views.
Unlock instant AI solutions
Tap the button
to generate a solution
to generate a solution
Click the button to generate
a solution
a solution
Knowledge Booster
Similar questions
- shift Humans have three types of cone cells in their eyes, which are responsible for color vision. Each type absorbs a certain part of the visible spectrum. Suppose a particular cone cell absorbs light with a wavelength of 542.nm. Calculate the frequency of this light. Round your answer to 3 significant digits. I Don't Know 84 tab THZ caps lock Type here to search Esc dd ! Submit 1 F1 Q A N Z 0x10 J @ 0 2 X F2 W S J #m F3 3 X S 1 E 9x D F4 $ 4 C R F F5 % 5 T V F6 N FO ( prt sc F10 9 1-²) K M 0 home O, O L < end F12 P 3 insert - = Danasia { L delete F olo Ar backspacearrow_forwardWhat is the wavelength (in nm) of T₂ given the information in the image? E₁ = ???? T₂= ???? E₂-4.56x10-19 J T₁ = 4.97x10-19 J E₁-7.33x10-19 Jarrow_forwardCalculate the frequency of light (in s1 also known as Hz) with a wavelength of (6.00x10^2) nm. Use a value of (3.0000x10^8) for the speed of light. Remember 1 m%3D (1.0000x10^9) nm Do not include units with your answer. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: х10 Answerarrow_forward
- The electron microscope has been widely used to obtain highly magnified images of biological and other types of materials. When an electron is accelerated through a particular potential field, it attains a speed of 4.00 106 m/s. The mass of the electron is 9.11 x 10-31 kg. Planck's constant has the value h = 6.63 x 10-34 J-s. What is the characteristic wavelength of this electron? ______marrow_forwardWhat is the uncertainty in its position? Express your answer with the appropriate units. Ar 2 Value Unitsarrow_forwardWhich color is closest to a wavelength of 5.0x10^-7 meter in air? Wavelength in a vacuum (m) 10-13 10-12 10 11 10 10 10 10 10 10 10 10 10³ 10² 10¹ 10⁰ 10¹ a C d Gamma Rays b violet e green E infrared orange red -X rays Ultraviolet Infrared 1021 1020 1019 108 107 106 105 10¹4 103 102 10¹ 101⁰ Frequency (Hz) Microwaves Visible Light (not to scale) Violet | Blue | Green | Yellow | Orange Red 10⁰ FM 10² 10³ 104 Radio Waves AM +Long Radio Waves -- 108 107 105 105arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY