
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
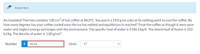
Transcribed Image Text:Incorrect.
An insulated Thermos contains 130 cm3 of hot coffee at 88.0°C. You put in a 19.0 g ice cube at its melting point to cool the coffee. By
how many degrees has your coffee cooled once the ice has melted and equilibrium is reached? Treat the coffee as though it were pure
water and neglect energy exchanges with the environment. The specific heat of water is 4186 J/kg-K. The latent heat of fusion is 333
kJ/kg. The density of water is 1.00 g/cm³.
Number
i
66.63
Units
C°
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A 0.310 kg aluminum bowl holding 0.795 kg of soup at 25.0°C is placed in a freezer. What is the final temperature (in °C) if 373 kJ of energy is transferred from the bowl and soup, assuming the soup's thermal properties are the same as that of water? °℃ tarrow_forwardIn an electrically heated home, the temperature of the ground in contact with a concrete basement wall is 12.4 oC. The temperature at the inside surface of the wall is 18.4 oC. The wall is 0.13 m thick and has an area of 8.4 m2. Assume that one kilowatt hour of electrical energy costs $0.10. How many hours are required for one dollar's worth of energy to be conducted through the wall?arrow_forwardA block of 10.0 kg of solid gold at its melting point of 1,063.00°C changes phase to liquid gold at 1,063.00°C and then changes phase back to solid gold at 1,063.00°C. The latent heat of fusion for gold is 6.44×104 J/kg. What is the total change in internal energy of the gold during this whole process?arrow_forward
- A chunk of hot iron is pulled out of a fire and placed between aluminum vice grips. The mass of the iron is 219 g and the mass of the aluminum is 66.4 g. The iron is initially 351.8 °C. The aluminum is initially 24.7 °C. Assuming no loss of heat to the environment, what is the final temperature of the iron-aluminum system? I couldn't even get started on this one.arrow_forwardThe temperature of a aluminum bar rises by 10.0°C when it absorbs 4.73 kJ of energy by heat. The mass of the bar is 525 g. Determine the specific heat of aluminum from these data.arrow_forwardYou have 975 grams of liquid water in a 275 gram glass container. Initially, the water and the container both have a temperature of 22.5 °C. You would like to cool the water and the container to a final temperature of 10.5 °C. You have a large supply of ice, which is initially at 0 °C. How much ice would you need to add to the container in order to get the desired final temperature after the ice melts and thermal equilibrium is reached? Give your answer in grams. The specific heat of liquid water is 4,186 J kg-1 °C-1, the specific heat of glass is 845 J kg-1 °C-!, and the latent heat of fusion of water is 333,500 J kg-!. Assume that the glass + liquid water + ice is an isolated system (i.e., no heat flows in or out of the system during this process).arrow_forward
- How much thermal energy (in J) is required to boil 2.45 kg of water at 100.0°C into steam at 135.0°C? The latent heat of vaporization of water is 2.26 ✕ 106 J/kg and the specific heat of steam is 2010 J kg · °C . HINT Jarrow_forwardA rectangular window in a home has a length of 1.5 m and a height of 0.80 m. If the window allows heat to escape from the home at a rate of 2,000 watts, how thick must the window be if the inside temperature of the home is 220 C and the outside temperature is 3.00C? (Assume that the coefficient of thermal conduction of glass is 0.80 W/mK.) a. 7.1 mm b. 124 mm c. 9.1 mm d. 8.1 mm e. 11 mmarrow_forwardThere is a .32 kg block of silver that is 21 °C. If silver's melting point is 961°C, how much thermal energy would a scientist need to melt this block of silver? (Hf = 111 kJ/kg). Hf is the heat of fusion of silver! Solve, assuming that the specific heat of silver is .24 J/g*degC, or 235 J/kg*K.arrow_forward
- A 6.25 kg block ice at 0 degrees Celsius is being warmed on a glass stove top. The thermal conductivity of the glass is 1.00 W/ (m K) and the glass is 0.50 cm thick. If the radiator plate underneath the glass raises the temperature of the bottom of the glass to 125 degrees Celsius, how long would it take to completely melt the ice? Assume the ice remains a solid rectangle with a square base of side 15 cm as it melts. B. What is the rate of entropy change in Joules/ Kelvin/ seconds of the melting ice?arrow_forwardA parcel of air with a volume of 9.3 x 10 km that contains 4.8 x 10 kg of water vapor rises to an altitude where all the water in the parcel condenses and then freezes. What is the change in temperature of the parcel of air due to freezing? Assume the density of air at the condensation altitude is 7.2 x 10 g/m. The specific heat of air is 0.17 cal/g Co, the latent heat of vaporization of water is 540 cal/g, and the latent heat of fusion of water is 80 cal/g.) Express the answer in standard seientific nötation. AT= x 10arrow_forwardA block of copper with mass 0.600 kg is heated to 400. degrees Celsius from 260. degrees Celsius. The specific heat capacity of copper is 387 J/ (kg°C). The heat absorbed by the copper block is 2.88 E5 J 3.25 E4 J 6.04 E5 J 9.29 E4 Jarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON