
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question
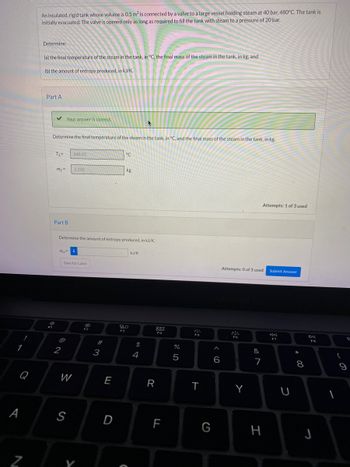
Transcribed Image Text:### Thermodynamic Problem Solving: Insulated Tank with Steam
#### Problem Statement
An insulated, rigid tank whose volume is \(0.5 \, \text{m}^3\) is connected by a valve to a large vessel holding steam at \(40 \, \text{bar}\), \(480°C\). The tank is initially evacuated. The valve is opened only as long as required to fill the tank with steam to a pressure of \(20 \, \text{bar}\).
Determine:
- (a) The final temperature of the steam in the tank, in °C, and the final mass of the steam in the tank, in kg.
- (b) The amount of entropy produced, in kJ/K.
#### Part A
**Determine the final temperature of the steam in the tank, \( T_2 \), and the final mass of the steam in the tank, \( m_2 \):**
- **\( T_2 \)**: \( 660.26 \, \degree \text{C} \)
- **\( m_2 \)**: \( 2.338 \, \text{kg} \)
#### Part B
**Determine the amount of entropy produced, \( \Delta S_{gen} \), in kJ/K:**
Input area is shown for data entry:
- **\( \Delta S_{gen} \)**: ________ \( \text{kJ/K} \)
(Submit Answer button shown for calculation verification)
#### Explanation
In Part A, the user correctly determined the final temperature of the steam as \(660.26 \, \degree \text{C}\) and the final mass of steam as \(2.338 \, \text{kg}\) based on the provided thermodynamic parameters and constraints.
In Part B, the process of determining the entropy change is initiated but the result has not yet been provided or confirmed.
This example illustrates the problem-solving approach to understanding the behavior of steam under specific conditions in thermodynamic processes, useful for engineering students studying fluid mechanics and thermodynamics.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- This Concepts revolves around thermodynamics and this practice example may need a PV diagram Directions are belowarrow_forwardThis question requires the use of the extracts from the tables of thermodynamic properties supplied. Ammonia, NH3, was once a commonly used refrigerant. In a particular (very old) refrigeration plant, ammonia has a pressure of 2.9 bar and a temperature of 40 °C. What would be the specific enthalpy of the ammonia under these conditions. 1433 kJ/kg a. b. 1552 kJ/kg 135 kJ/kg O d. 1622 kJ/kg O e. 1665 kJ/kgarrow_forwardDiscuss all the laws of thermodynamics (Zeroth, first, second and third) and give their physical significance.arrow_forward
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY