
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
An ideal gas goes through a series of dynamically reversible processes in a closed system. Calculate W, Q, ∆U, and ∆H for each of the following three processes and for the entire cycle. The heat capacity Cv=3/2 R of the ideal gas is set to Cp=5/2 R (25).
(a) It is adiabatically compressed up to 150°C in the initial state of 70°C and 1 bar.
(b) Next, it is cooled from 150°C to 70°C under constant pressure. (c) Finally, it expands isothermal to its initial state again.
Expert Solution
arrow_forward
PV diagram and the state variables
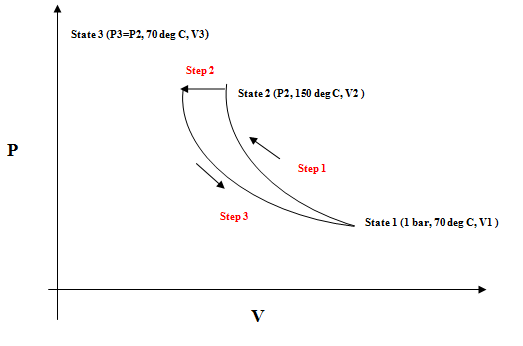
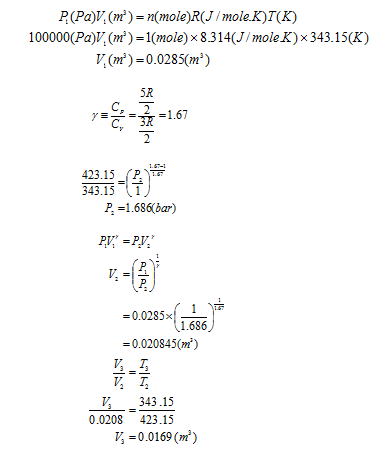
Step by stepSolved in 4 steps with 5 images

Knowledge Booster
Similar questions
- Heat in the amount of 7.5 kJ is added to a closed system while its internal energy decreases by 12 kJ. a) How much energy is transferred as work? b) For a process causing the same change of state but for which the work is zero, how much heat is transferred?arrow_forwardConsider an ideal gas which is being throttled to a pressure of 1 bar at a rate of 100 mol/s The initial pressure of the system is 25 bar. Given the temperature of the surrounding as 300 K. Calculate the following quantities (a) Rate of entropy generation Lost workarrow_forward11-14. The mixture in Problem 11-1 is contained in a rigid vessel and is heated to a temperature of 120°C by transferring heat from a thermal-energy reservoir having a temperature of 300°C. The volume of the vessel is 0.4 m³, and the environment is at 25°C and 100 kPa. Determine the amount of heat transfer required.arrow_forward
- Very good system. There is a 100 It capacity capacitive 25°C nitrogen gas at 200 kPa pressure. This valve throttles in electricity consumption at 90°C. The connection is opened and the pressure in the container is filled with its use until it becomes 400 kPa. Find the final temperature and total entropy in the container with constant specific heats. NOTE: The quadratic solution with ax2+bx+c=0 is c=0; -b + vb2 – 4ac x = 2aarrow_forwardA piston–cylinder device contains 0.05 kg of steam at 1 MPa and 300°C. Steam now expands to a final state of 200 kPa and 150°C, doing work. Heat losses from the system to the surroundings are estimated to be 2 kJ during this process. Assuming the surroundings to be at T0 = 25°C and P0 = 100 kPa, determine the exergy change of the steam.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The