
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
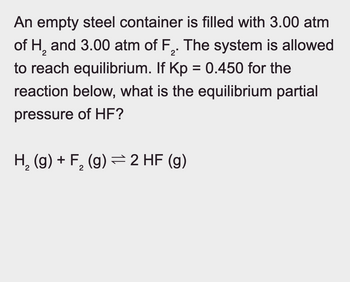
Transcribed Image Text:An empty steel container is filled with 3.00 atm
of H2 and 3.00 atm of F2. The system is allowed
to reach equilibrium. If Kp = 0.450 for the
reaction below, what is the equilibrium partial
pressure of HF?
H2(g) + F₂ (g) 2 HF (g)
2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Consider the following reaction:2SO3(g) 2SO2(g) + O2(g)If 8.04×10-2 moles of SO3, 0.396 moles of SO2, and 0.261 moles of O2 are at equilibrium in a 19.8 L container at 1.31×103 K, the value of the equilibrium constant, Kp, isarrow_forwardConsider the equilibrium system described by the chemical reaction below. At equilibrium, a 2.0 L reaction vessel contained a mixture of 1.0 mol Fe, 1.0 × 10⁻³ mol O₂, and 2.0 mol of Fe₂O₃ at 2000 °C. What are the values of Kc and Kp for this reaction? 4 Fe(s) + 3 O₂(g) ⇌ 2 Fe₂O₃(s) Based on your value of Kc, determine the value of Kp under these conditions.arrow_forwardAn empty steel container is filled with 0.840 atm of A and 0.840 atm of B. The system is allowed to reach equilibrium according to the reaction below. If Kp = 340 for this reaction, what is the equilibrium partial pressure of C? A (g) + B (g) = C (g)arrow_forward
- An empty steel container is filled with 2.50 atm of H₂ and 2.50 atm of F₂. The system is allowed to reach equilibrium. If Kp = 0.450 for the reaction below, what is the equilibrium partial pressure of HF? H₂ (g) + F₂ (g) ⇌ 2 HF (g)arrow_forwardAn empty steel container is filled with 0.0410 atm of HF. The system is allowed to reach equilibrium. If Kp = 2.76 for the reaction below, what is the equilibrium partial pressure of H₂? 2 HF (g) ⇌ H₂ (g) + F₂ (g)arrow_forwardConsider the following reaction where K = 77.5 at 600 K. CO(g) + Cl₂(g) 2 CoCl₂(g) A reaction mixture was found to contain 1.89×10-² moles of CO(g), 4.43×10-² moles of Cl₂(g) and 0.106 moles of COCI₂(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qc, equals The reaction A. must run in the forward direction to reach equilibrium. B. must run in the reverse direction to reach equilibrium. C. is at equilibrium.arrow_forward
- An empty steel container is filled with 0.0710 atm of HF. The system is allowed to reach equilibrium. If Kp = 2.76 for the reaction below, what is the equilibrium partial pressure of H₂? 2 HF (g) ⇌ H₂ (g) + F₂ (g)arrow_forwardConsider the reaction. 2 A(g) = B(g) Kp = 5.62 x 10-5 at 500 K If a sample of A(g) at 4.20 atm is heated to 500 K, what is the pressure of B(g) at equilibrium? PB = atmarrow_forwardAn empty steel container is filled with 6.10 atm of H₂ and 6.10 atm of F₂. The system is allowed to reach equilibrium. If Kp = 0.450 for the reaction below, what is the equilibrium partial pressure of HF? H₂ (g) + F₂ (g) ⇌ 2 HF (g)arrow_forward
- An empty steel container is filled with 2.60 atm of H₂ and 2.60 atm of F₂. The system is allowed to reach equilibrium. If Kp = 0.450 for the reaction below, what is the equilibrium partial pressure of HF? H₂ (g) + F₂ (g) ⇌ 2 HF (g)arrow_forwardA reaction vessel is charged with 0.50 atm of A and 0.300 atm of B. Once the reaction reaches equilibrium, what is the equilibrium partial pressure of B? Kp for this reaction is 67.2 A(g) - 2 B (g)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY