
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
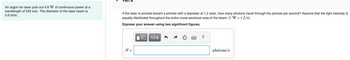
Transcribed Image Text:An argon ion laser puts out 4.8 W of continuous power at a
wavelength of 532 nm. The diameter of the laser beam is
5.6 mm.
If the laser is pointed toward a pinhole with a diameter of 1.2 mm, how many photons travel through the pinhole per second? Assume that the light intensity is
equally distributed throughout the entire cross-sectional area of the beam. (1 W = 1 J/s)
Express your answer using two significant figures.
N =
—| ΑΣΦ
?
photons/s
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Infrared radiation from young stars can pass through the heavy dust clouds surrounding them, allowing astronomers here on Earth to study the earliest stages of star formation, before a star begins to emit visible light. Suppose an infrared telescope is tuned to detect infrared radiation with a frequency of 13.6THZ. Calculate the wavelength of the infrared radiation. Round your answer to 3 significant digits. umarrow_forwardImagine an alternate universe where the value of the Planck constant is 6.62607 × 10-40 J.S. In that universe, which of the following objects would require quantum mechanics to describe, that is, would show both particle and wave properties? Which objects would act like everyday objects, and be adequately described by classical mechanics? object A raindrop with a mass of 3.0 mg, 0.8 mm wide, moving at 6.6 m/s. An eyelash mite with a mass of 9.1 µg, 260 pm wide, moving at 44. um/s. An alpha particle with a mass of 6.6 x 10-27 kg, 8.0 x 10 15 m wide, moving at 16. km/s. A car with a mass of 1300. kg, 4.6 m long, moving at 134.0 km/h. quantum or classical? classical O quantum oooooo O classical O quantum O classical O quantum O classical O quantum X Sarrow_forwardBe sure to answer all parts How fast must a 143.9−g baseball travel in order to have a de Broglie wavelength that is equal to that of an x-ray photon with λ= 75.0 pm? Enter your answer in scientific notation please.arrow_forward
- What is the de Broglie wavelength of an oxygen molecule, O_(2), traveling at 521(m)/(s) ? Is the wavelength much smaller or much larger than the diameter of an atom (on the order of 100pm)?arrow_forwardHow fast must a 132 g baseball travel in order to have a de Broglie wavelength that is equal to that of an x-ray photon with A = 100. pm? 4.0 m/sarrow_forwardA laser pointer is found to emit light with a frequency of 4.93 x 1014 Hz. Determine the wavelength (in nm) of a photon from this laser pointer. For large numbers, you can leave off the "x 10" portion here. For example, if you answer is 5.5 x 1025, you can type "5.5" here and write the full answer on your paper.arrow_forward
- Enormous numbers of microwave photons are needed to warm macroscopic samples of matter. A portion of soup containing 445 g of water is heated in a microwave oven from 20.0°C to 98.0°C, with radiation of wavelength 1.55x10−2 m. How many photons are absorbed by the water in the soup?arrow_forwardThe relationship between photons of light at various energies and wavelengths is Given by E=hv , c=v(wavelength) Therefore, when a photon of energy at wavelength of 275 nm is compared with photons of energy at wavelength of 390 nm and higher we have a decrease in the energy and its dissociation energy that would attack polymeric or chemical bonds of various structures. Therefore, the higher the wavelength the lower the frequency or higher wavelengths have less energy than lower wavelengths 275nm is far greater energy than 390nm or higher. Given we have established light is composed of photons and high energy gamma radiation are also photons but of higher energy does the math for photochemistry given above apply to higher sources of photon energy from gamma and other sources of radiation? If so why or why not. If there is a relationship what equations exist to compare and predict equivalence of standard electromagnetic model wavelength for actinic and visible and IR radiations to more…arrow_forwardCalculate the kinetic energy of an electron ejected from the surface of a sample of Rubidium (binding energy = 2.26 eV (where 1 eV = 1.60218 x 10-19 J) when the metal is subjected to photons having a wavelength of 325 nm.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY