
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
need help with this chemistry review and can pls be clear with step by step
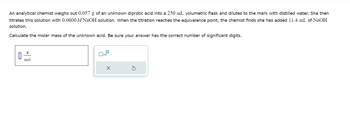
Transcribed Image Text:An analytical chemist weighs out 0.057 g of an unknown diprotic acid into a 250 mL volumetric flask and dilutes to the mark with distilled water. She then
titrates this solution with 0.0600 M NaOH solution. When the titration reaches the equivalence point, the chemist finds she has added 11.4 mL of NaOH
solution.
Calculate the molar mass of the unknown acid. Be sure your answer has the correct number of significant digits.
0
g
|
mol
0
x10
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. All chromatography involves a solid phase, and mobile phase. In thin layer chromatography the solid phase is the silica layer on a piece of glass, and the mobile phase is the organic solvent that will travel along the TLC plate. which direction will the mobile phase (the organic solvent) travel? A) UPWARD B) DOWNWARD C) HORIZONTALLY LEFT TO RIGHTarrow_forward12: Mastery - Kinetics X com/ilm/takeAssignment/takeCovalentActivity.do?locator=assignment-take juju_ ncepts " Q Search OWLv2 | Online teaching and le Free Online Survey... [CO] = [Cl₂] = [COCI₂] = Submit Answer Home - Academia... PRO Modules Create Your Rubric-... [Review Topics] [References] Use the References to access important values if needed for this question. The equilibrium constant, K, for the following reaction is 77.5 at 600 K. CO(g) + Cl₂(g) CoCl₂(g) Calculate the equilibrium concentrations of reactant and products when 0.474 moles of CO and 0.474 moles of Cl₂ are introduced into a 1.00 L vessel at 600 K. Retry Entire Group IL & M M M X + hp 4+ 7 more group attempts remaining 8 19 ( 9 110 The Utility Experts ... m 4 12 = insert » | ← 12/1 baarrow_forward1. List the concentrations of the stock. [Acetone] 0.6M [lodine] 00025M [HC1] 2. From the computer plot of the data for each trial (absorbance vs. time in seconds), detemine the slope of the best straight line; use the following data table to summarize your results: Table 1. Trial # Acetone, mL lodine, mL HCI, mL AAbs/At ( slope of line) H20, mL A 1.0 1.0 1.0 2.0 -0.0010258xto:34978. t0.0016339x+0.44357 70,00g0217x 0.89112 0. 0013205xto,235271 2.0 1.0 1.0 1.0 1.0 2.0 1.0 1.0 D 1.0 1.0 2.0 1.0 3. Convert stock concentrations to initial concentrations and fill in Table 2: Table 2. Trial # Disappearance Rate of l2 (M/s) -A[L/At (= -slope/730) [Acetone] [lodine] [HCIJ 0.12 0.00050.12 0.24 0.12 10.12 0. coe 5 0.12 0.001 0.12 D 0.00050.24arrow_forward
- One disadvantage of thin layer chromatography is that... it cannot be used for quantitative measurements. O it consumes large volumes of solvents O it cannot analyze volatile samples it cannot be used to analyze many samples simultaneously it requires a large amount of sample for analysisarrow_forwardOrganic Chemistry, column questionsarrow_forwardI do not know how to find the molecular formulaarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY