
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
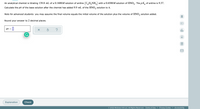
Transcribed Image Text:An analytical chemist is titrating 139.8 mL of a 0.1600M solution of aniline (C,H,NH,) with a 0.6500M solution of HNO,. The p K, of aniline is 9.37.
Calculate the pH of the base solution after the chemist has added 9.9 mL of the HNO, solution to it.
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO, solution added.
Round your answer to 2 decimal places.
pH
olo
G)
18
Ar
Explanation
Check
© 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An analytical chemist is titrating 208.8mL of a 0.7500M solution of methylamine CH3NH2 with a 0.5600M solution of HNO3 . The pKb of methylamine is 3.36 . Calculate the pH of the base solution after the chemist has added 236.8mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places.arrow_forwardA chemist titrates 160.0 mL of a 0.3445 M pyridine (C-H5N) solution with 0.8552M HNO3 solution at 25 °C. Calculate the pH at equivalence. The pK, of pyridine is 8.77. 6 Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HNO3 solution added. E pH = 0 X 5 OL 9 Larrow_forwardAn analytical chemist is titrating 137.4 mL of a 0.2300M solution of methylamine (CH3NH2) with a 0.1600M solution of HNO3. The pK of methylamine is 3.36. Calculate the pH of the base solution after the chemist has added 223.0 mL of the HNO3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added. Round your answer to 2 decimal places.arrow_forward
- For each of the salts (assume at this point completely soluble), write the equation for dissociation and label the ions as acidic, basic or neutral.Each salt listed will 100% dissociate NH4C2H3O2 Al2(CO3)3 Na2HPO4 NaI For each of the following salts, determine the pH of a 2.50M solution of the salt.You will need the Ka and Kb tables to complete this problem. NaBrO CH3NH3Cl NaClO2 C6H5OK CsNO3 C6H5NH3Brarrow_forwardAn analytical chemist is titrating 135.0 mL of a 0.06400M solution of piperidine (CH,NH) with a 0.4700M solution of HNO3. The pK, of piperidine is 2.89. Calculate the pH of the base solution after the chemist has added 21.3 mL of the HNO 3 solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO 3 solution added. Round your answer to 2 decimal places. pH = 0 ×arrow_forwardA chemist titrates 180.0 mL of a 0.3545M ammonia (NH3) solution with 0.7205M HBr solution at 25 °C. Calculate the pH at equivalence. The pk, of ammonia is 4.75. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. -0 pH = Xarrow_forward
- An analytical chemist is titrating 119.2 mL of a 0.1800M solution of ammonia (NH,) with a 0.4500M solution of HNO2. The p Kb of ammonia is 4.74. Calculate the pH of the base solution after the chemist has added 53.9 mL of the HNO, solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO, solution added. 3 Round your answer to 2 decimal places. pH ?arrow_forwardAn analytical chemist is titrating 109.3 ml. of a 0.8700 M solution of butanoic acid (HC,H,CO,) with a 0.9500 M solution of NaOH. The pK, of butanoic add is 4.82. Calculate the pH of the and solution after the chemist has added 36.93 mL of the NaOH solution to it. Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of NaOH solution added. Round your answer to 2 decimal places. pH = 0 Xarrow_forwardA chemist titrates 90.0 mL of a 0.7050M methylamine (CH3NH₂) solution with 0.5016M HBr solution at 25 °C. Calculate the pH at equivalence. The pK, of methylamine is 3.36. Round your answer to 2 decimal places. Note for advanced students: you may assume the total volume of the solution equals the initial volume plus the volume of HBr solution added. pH = x 5 ?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY