
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:E
www-awu.aleks.com/alekscgi/x/
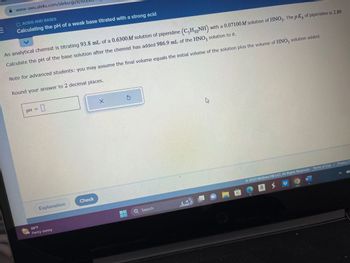
O ACIDS AND BASES
Calculating the pH of a weak base titrated with a strong acid
An analytical chemist is titrating 93.8 mL of a 0.6300M solution of piperidine (C5H₁NH) with a 0.07100M solution of HNO3. The pK, of piperidine is 2.89.
Calculate the pH of the base solution after the chemist has added 986.9 mL of the HNO3 solution to it.
Note for advanced students: you may assume the final volume equals the initial volume of the solution plus the volume of HNO3 solution added.
Round your answer to 2 decimal places.
pH =
Explanation
66°F
Partly sunny
Check
X
S
Q Search
AS
B
W
UNDER
© 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce
CEREDARE FR
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- QUES TION 4 The pH value is an expression of the molarity of H30 ions in solution. This concentration has strong impact on chemical reactions. The molarity is very small value, and therefore awkward to use. The pH scale simplifies this concentration making communication easier and faster. Demonstrate the relationship between pH and H30 molarity, using the formula, pH = -log(H30) where ) indicates concentration in molarity of a substance. What is the pH of a solution, having the H30* concentration of 0.00014 M?arrow_forwardWhat is the pH of a solution that is 0.57 M in sodium fluoride and 0.69 M in hydrofluoric acid? Report your answer to 2 decimal places. Your Answer: Answerarrow_forwardCalculate the pH and pOH of the following solutions :1- 0.01 M HBr2- 0.00005 M HBr3- 0.00002 M KOH4- 0.01 M CH COOH5- 0.00001 M CH,COOH6- 0.01 M HIO,7-0.01 M pyridinearrow_forward
- #8 - thumbs up if you do it correctly! Be sure your answer has the correct number of significant digits as it states in the directions.arrow_forwardPlease answer the following, if you have a different answer please specify what you got.arrow_forwardI really need help on this question please help I really need it!arrow_forward
- An engineer wants to determine whether a certain pH meter is working correctly. She uses the meter to measure the pH in 14 neutral substances (pH = 7.0) and obtains the data shown in the following table. 7.01 7.04 6.97 7.00 6.99 6.97 7.04 7.04 7.01 7.00 6.99 7.04 7.07 6.97 Is there sufficient evidence that the meter is not correctly measuring the pH?arrow_forwardThis is a multi- answer question. Please type your properly rounded answers in the next three questions ( starting with this one):Calculate the pH of a 0.70 M solution of sodium sulfite, Na2SO3. Also calculate the concentrations of HSO3 and H2SO3. Type the properly rounded pH below:arrow_forwardWhat pH range is considered to be acidic? Basic? Neutral? Which pollutants are responsible for acid rain? Where do they come from?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY