
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
|
An airplane has a mass of 2.1×106 kgkg , and the air flows past the lower surface of the wings at 81 m/sm/s .
If the wings have a surface area of 1100 m2m2 , how fast must the air flow over the upper surface of the wing if the plane is to stay in the air?
Express your answer to two significant figures and include the appropriate units.
|
|
Transcribed Image Text:An airplane has a mass of 2.1×106 kg , and the air
flows past the lower surface of the wings at 81 m/s
Part A
If the wings have a surface area of 1100 m2 , how fast must the air flow over the upper surface of the wing if the
plane is to stay in the air?
Express your answer to two significant figures and include the appropriate units.
H HẢ
?
Vtop =
Value
Units
Expert Solution
arrow_forward
Step 1
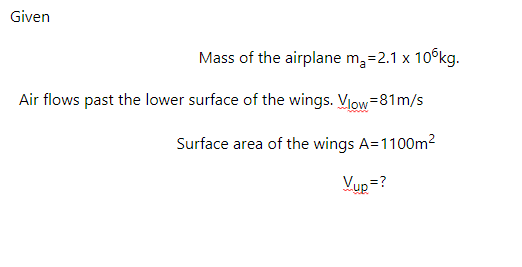
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A rock with mass 254 grams is found to displace 199 cubic centimeters of water when fully immersed. What is its (the rock's) density in kilograms per cubic meter? Don't put the unit; just the number.arrow_forwardThe density of the atmosphere varies drastically with height, but you can use an average density from sea floor to the space in order to calculate its height. If we approximate this average density to be = 1 Kg/m³, what would be height of the atmosphere? HINT: Since the atmosphere is a fluid (in gas form), you can use the hydrostatic pressure formula (P pgh), just solve for h and use P = 1 atm. BTW, this is how the edge of space is defined, if you make it pass the height of the atmosphere you have reached space :)arrow_forwardA cylinder containing 100.9 cubic centimeter of gas at a pressure of 376 kPa when its temperature is 534 K. Given that its temperature is unchanged when the pressure was increased by a factor of 5.9, Determine the new volume of the gas (In cubic centimeter). Note: Your answer must be in cubic centimeter, however, do not include the unit, just enter the magnitude that corresponds to the final volume in cubic centimeter. Round your answer to 2 decimal points Round your answer to 2 decimal pointsarrow_forward
- During the summer, the height of the water in a pool decreases by 4.25 inches each week due to evaporation. What is the change in the height of the water over a six-week period?arrow_forwardThe atmospheric pressure at the time the video was taken was recorded by the National Weather Service as 30.10 inches of mercury, with the alternative measure of a sea level equivalent 1018.8 bar. What was the atmospheric pressure in the laboratory (we are only a few km from the Weather Service's station) in SI units? The standard unit of pressure is the pascal. For atmospheric pressure it is conventional to use kPa, kilopascal. Why is there a difference between the measured pressure locally and the sea level equivalent, apart from the continued use of imperial units for barometric pressure.arrow_forwardThe reservoir in Figure contains water and immiscible oil at a temperature of 20 ° C. What is the height h in centimeters if the density of the oil is 898 kg / m3?arrow_forward
- A 5 cubic meter container is filled with 840kg of granite[density is 2400 kg per cubic meter] and the rest of the volume is air[density is 1.15 kg per cubic meter]. From the above statement: Find the mass of air present in the container. * 5.7534 kg 5.3475 kg 5.5374 kg O 5.4375 kgarrow_forwardAs blood passes through the capillary bed in an organ, the capillaries join to form venules (small veins). 1. If the blood speed increases by a factor of 4.00 as it enters the venules, which have a total cross-sectional area 9.5 cm2, what is the total cross-sectional area, in square centimeters, of the capillaries feeding these venules? A2=(in cm^2) 2. How many capillaries are involved if their average diameter is 7.5 μm? N=arrow_forwardAssuming that each cubic centimeter of water has a mass of exactly 1 g, find the mass of 8.70 cubic meter of water. (b) Suppose that it takes 15.0 hours to drain a container of 39.4 m3 of water. What is the "mass flow rate," of water from the container?arrow_forward
- The tidal lung volume of human breathing, representing the amount of air inhaled and exhaled in a normal breath, is 500 cm³. (Assume atmospheric pressure.) (a) What is the number of molecules of air inhaled with each human breath when the air temperature is 25.0°C? molecules (b) If the molar mass of air is 28.96 g/mol, what is the mass (in g) of air molecules inhaled with each breath? (Assume the air temperature is 25.0°C.) g (c) It has been calculated that all of the air in Earth's atmosphere could be collected into a sphere of diameter 1,999 km at a pressure of 1.00 atm. What is the mass (in kg) of the air in Earth's atmosphere? (Assume the density of air used in this calculation was 1.225 kg/m³.) kg (d) If all 7 billion humans on Earth inhaled simultaneously, what percentage of the atmosphere would be inhaled during this process? (Assume the air temperature is 25.0°C everywhere on Earth.) %arrow_forwardA conventional oil barrel (cylindrical in shape) has an internal diameter of 22 inches and a maximum external diameter of 23 inches. The height inside the barrel is 33 inches, with a maximum external height of of 34 inches. if only 92% of the volume of the barrel can be filled, what is this volume in liters?arrow_forwardWhat mass of silver has a volume of 150 cm3? A certain gas has a volume of 600 cm3. What is its volume when the pressure is tripled? Consider the gas in # 5 above. What is its volume if the pressure is changed to one-third its original value?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON