
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
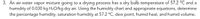
Transcribed Image Text:3. An air-water vapor mixture going to a drying process has a dry bulb temperature of 57.2 °C and a
humidity of 0.030 kg H2O/kg dry air. Using the humidity chart and appropriate equations, determine
the percentage humidity, saturation humidity at 57.2 °C, dew point, humid heat, and humid volume.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- Calculate and report the density, in kg m-³, of air at 1.00 atm and 10.0 °C. Take the composition of air to be 79 mol% nitrogen and 21.0 mol% oxygen. The molecular weights of nitrogen and oxygen may be taken as 28.0 g mol-¹ and 32.0 g mol-¹ respectively. State any assumptions.arrow_forwardIn Hurricane Andrew, the core temperature was about 5°C warmer than the surroundings. Some have suggested that detonating a bomb inside the storm could raise the temperature of the surroundings and reduce the intensity of it. How much energy would need to be released to raise the temperature by 5°C within an 80 km radius of the center up to a height of 1 km? Assume that the air density is 1.2 kg m^-3 and the air is dry (Cv= 718 J/kgK). Perform the calculation for a) isobaric heating and b) isosteric heating. Express your answers in both Joules and megatons.arrow_forwardThe result of air temperature measurement obtained dry ball temperature 37°C and wet ball temperature 27.5 °C. Using a graph of psychotry, determine the properties of the air as follows:a. RH : %B. Moisture content : kg of water / kg of airc. Specific volume : m3/kgd. Enthalpi : kJ/kgE. Condensation temperature : °CIf the air is within 162 m3 of space, specify theF. Weight of air (dry air and moisture) : Kgg. amount of water content in the room: kgarrow_forward
- A rectifying column consists of a tube arranged vertically and supplied at the bottom with a mixture of benzene and toluene as vapor. At the top, a condenser returns some of the product as a reflux which flows in a thin film down the inner wall of the tube. At one point in the column, the vapor contains 60 mol% benzene and the adjacent liquid reflux contains 51 mol% benzene. The temperature at this point is 92°C. Assuming the diffusional resistance to vapor transfer to be equivalent to the diffusional resistance of a štagnant vapor layer 0.5 mm thick, calculate the rate of interchange of benzene and toluene between vapor and liquid. The molar latent heats of the two materials can be taken as equal. The vapour pressure of toluene at 92°C is 44.0 kN/m2 and the diffusivity of the vapors is 0.053 cm?/s.arrow_forwardMETAL Suppose you heat a metal object with a mass of 32.9 g to 96.6 °C and transfer it to a calorimeter containing 100.0 g of water at 17.2 °C. The water and metal reach a final temperature of 22.4 °C. What is the specific heat of the metal in J/g-°C?arrow_forward17D.2. When a sample of gold iscooled from 32.5 °C to 20.0 °C,9.46 J of heat are liberated. What isthe mass of the sample if the specificheat of gold is 0.129 J/g·°C?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The