
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
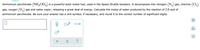
Transcribed Image Text:Ammonium perchlorate \((\text{NH}_4\text{ClO}_4)\) is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen \((\text{N}_2)\) gas, chlorine \((\text{Cl}_2)\) gas, oxygen \((\text{O}_2)\) gas and water vapor, releasing a great deal of energy. Calculate the moles of water produced by the reaction of 2.0 mol of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.
There is a chemical equation with interactive selectable symbols, including arrows for reversing and resetting the calculation, and a question mark for help.
No graphs or diagrams are present.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Ammonlum perchlorate (NH, CIO Is a powerful solld rocket fuel, used in the Space Shuttle boosters. It decomposes Into nitrogen (N,) gas, chlorine (Cl,) gas, oxygen (0,) gas and water vapor, releasing a great deal of energy. Calculate the moles of nitrogen produced by the reaction of 0.10 mol of ammonium perchlorate. Be sure your answer has a unlt symbol, If necessary, and round it to 2 significant digits. Submit Assignn Continue © 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use| Privacy Center| Acces escarrow_forwardCalculate the moles of chlorine produced by the reaction of 0.065mol of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits.arrow_forwardAmmonium perchlorate (NH CIO.) is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen (N,) gas, chlorine (Cl,) gas, oxygen (0,) gas and water vapor, releasing a great deal of energy. Calculate the moles of water produced by the reaction of 0.050 mol of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to 2 significant digits. x10 Ib Proj I Don't Know Submit © 2020 McGraw-Hill Education. All Rights Reserved. Terms of Use l Privacy JAN 10 10 tv MacBook Pro esc Thank Yeah %24 3 T. H. в 36 on command option command 00 C.arrow_forward
- Ammonium perchlorate NH4ClO4 is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen N2 gas, chlorine Cl2 gas, oxygen O2 gas and water vapor, releasing a great deal of energy. Calculate the moles of oxygen produced by the reaction of 0.075mol of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.arrow_forwardUsing a chemical equation to find moles of product from moles... Wine goes bad soon after opening because the ethanol (CH3CH₂OH) dissolved in it reacts with oxygen (O₂) gas to form water and aqueous acetic acid (CH₂COOH), the main ingredient in vinegar. Calculate the moles of water produced by the reaction of 2.4 mol of ethanol. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits. 0 Explanation Check 010 4 X 0.0 eveu muGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centearrow_forwardAmmonium perchlorate (NH4CIO4) is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen (N2) gas, chlorine (Cl2) gas, oxygen (02) gas and water vapor, releasing a great deal of energy. Calculate the moles of water produced by the reaction of 2.1 mol of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.arrow_forward
- For the following reaction, 14.2 grams of carbon monoxide are allowed to react with 5.87 grams of oxygen gas . carbon monoxide(g) + oxygen(g) → carbon dioxide(g) What is the maximum mass of carbon dioxide that can be formed? grams What is the FORMULA for the limiting reagent? What mass of the excess reagent remains after the reaction is complete? grams Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardNonearrow_forwardAmmonium perchlorate (NH4ClO4) is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen (N2) gas, chlorine (Cl2) gas, oxygen (O2) gas and water vapor, releasing a great deal of energy. Calculate the moles of ammonium perchlorate needed to produce 1.2mol of water. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.arrow_forward
- Please see imagearrow_forwardAmmonium perchlorate (NH4CIO) is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen (N₂) gas, chlorine (C1₂) gas, oxygen (0₂) gas and water vapor, releasing a great deal of energy. Calculate the moles of chlorine produced by the reaction of 0.900 mol of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to 3 significant digits. 1 010 7 X ロ・ロ 3arrow_forwardFor the following reaction, 5.63 grams of methane (CH4) are allowed to react with 25.0 grams of carbon tetrachloride . methane (CH4)(g) + carbon tetrachloride(g) –→ dichloromethane (CH,Cl,)(g) What is the maximum mass of dichloromethane (CH,Cl,) that can be formed? grams What is the FORMULA for the limiting reagent? What mass of the excess reagent remains after the reaction is complete? grams Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY