
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
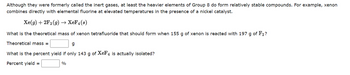
Transcribed Image Text:Although they were formerly called the inert gases, at least the heavier elements of Group 8 do form relatively stable compounds. For example, xenon
combines directly with elemental fluorine at elevated temperatures in the presence of a nickel catalyst.
Xe(g) + 2F₂(g) → XeF4(s)
What is the theoretical mass of xenon tetrafluoride that should form when 155 g of xenon is reacted with 197 g of F2?
Theoretical mass=
g
What is the percent yield if only 143 g of XeF4 is actually isolated?
Percent yield =
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Chlorine gas can be produced in the laboratory by adding concentrated hydrochloric acid, HCl, to manganese(IV) oxide according to the following reaction: MnO2 (s) + 4 HCl (aq) → MnCl2 (aq) + 2 H2O (l) + Cl2 (g) What mass of MnO2 is required to produce 48.4 g Cl2 by the following reaction? Be sure to enter a unit with your answer.arrow_forwardDisulfur dichloride, which has a revolting smell, can be prepared by directly combining Se and Cl₂, but it can also be made by this reaction: 3 SC1₂ (1) + 4 NaF (s) → SF4 (g) + S₂ Cl₂ (l) + 4 NaCl(s) Calculate the mass of SC12 needed to react with excess NaF to prepare 1.37 g S₂ Cl2 if the expected yield is 46%. Mass of SC12 = garrow_forward4 KO₂ (s) + 2 CO₂(g) → 2 K₂CO₃(s) + 3 O₂(g) What is the mass in grams of oxygen gas that can be produced from 0.568 grams of KO₂?arrow_forward
- The Ostwald process is used commercially to produce nitric acid, which is, in turn, used in many modern chemical processes. In the first step of the Ostwald process, ammonia is reacted with oxygen gas to produce nitric oxide and water. What is the maximum mass of H,O that can be produced by combining 81.4 g of each reactant? 4 NH, (g) + 50,(g) 4 NO(g) + 6 H, O(g) > g H,O mass:arrow_forwardCryolite (Na3AlF6) is used in the production of aluminum from its ores. It is made by the reaction 6 NaOH + Al2O3 + 12 HF → 2 Na3AlF6 + 9 H2O Calculate the mass of cryolite that can be prepared by the complete reaction of 287 g Al2O3.arrow_forwardWhat is the mass in grams of H₂ that can be formed from 88.2 grams of NH, in the following reaction? 3 2 NH₂(g) → 3 H₂(g) + N₂(g)arrow_forward
- How many moles of Al are necessary to form 52.2 g of AlBr₃ from this reaction: 2 Al(s) + 3 Br₂(l) → 2 AlBr₃(s) ?arrow_forwardThe Ostwald process is used commercially to produce nitric acid, which is, in turn, used in many modern chemical processes. In the first step of the Ostwald process, ammonia is reacted with oxygen gas to produce nitric oxide and water. What is the maximum mass of H,O that can be produced by combining 84.6 g of each reactant? 4 NH,(g) + 5 0,(g) → 4 NO(g) + 6 H,O(g) g H,O mass: 61 Sunnyarrow_forwardIf 9.9 × 10²⁵ molecules of CO₂ are produced in a combustion reaction, what is the mass in kg of CO₂ that is produced?arrow_forward
- Consider the reaction of solid P₄ and chlorine gas to form gaseous phosphorus trichloride. The balanced chemical equation is P₄(s) + 6 Cl₂(g) → 4 PCl₃(g). How many grams of phosphorus trichloride can be formed from 419.7 grams of P₄ based on the balanced chemical equation?arrow_forwardThe Ostwald process is used commercially to produce nitric acid, which is, in turn, used in many modern chemical processes. In the first step of the Ostwald process, ammonia is reacted with oxygen gas to produce nitric oxide and water. What is the maximum mass of H₂O that can be produced by combining 50.1 g of each reactant? 4 NH3(g) +50₂(g) -> 4 NO(g) + 6H₂O(g)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY