
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
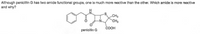
Transcribed Image Text:Although penicillin G has two amide functional groups, one is much more reactive than the other. Which amide is more reactive
and why?
CH3
CH3
COOH
penicillin G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 17-12 Classify each of the following amines as a primary, secondary, or tertiary amine. a. CH3-CH2-CH-CH₂—CH₂ NH₂ b. CH3 CH3-C-CH3 NH₂ C. CH3-N-CH3 CH3 d. CH—CHNH–CH–CH, CH3 CH3 17-arrow_forwardNitriles can be formed by treating primary and secondary amides secondary amines primary amines with SOCl2? tertiary aminesarrow_forwardWhich is a primary amine? O(CH₂CH₂)2N O (CH3)2NH O (CH3)3CNH2 O(CH₂4N CTarrow_forward
- What is the amide formed in the following reaction?arrow_forwardClassify the following amine: NH₂ Primary (1°) amine Secondary (2°) amine Tertiary (3°) amine Quaternary (4°) ammoniumarrow_forwardWhat is the name of the following structure? N-methylpentan amide N-methylpentan-2-amine N-methylpentyl-2amine N-methyl-N-1-mentylaminobutanearrow_forward
- O which reagents can reduce amide? 1. LIAIHY 2. H20, H30* ETOHarrow_forwardIdentify TWO substrate and one reagent that could be combined to make the completed product.arrow_forwardWhich of the following amines will not show intermolecular hydrogen bonding? a) CH3NH2 b) (CH3)2NH c) (CH3)3N d) all of thesearrow_forward
- Is this a primary , secondary , tertiary amine. Or a quaternary ammonium salt?arrow_forwardClassify the following amides as primary, secondary, or tertiary amides. Write NR if the compound is not an amide. Please explainarrow_forwardBenzodiazepines are known to have a wide therapeutic window. What makes benzodiazepines relatively safe even when consumed in higher than therapeutic doses?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY