
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
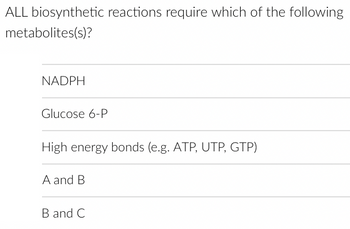
Transcribed Image Text:ALL biosynthetic reactions require which of the following
metabolites(s)?
NADPH
Glucose 6-P
High energy bonds (e.g. ATP, UTP, GTP)
A and B
B and C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Give one of the names for the pathway shown below.arrow_forwardAn organic cofactor (usually derived from a vitamin or a nucleotide), which is tightly attached to the enzyme (like FAD when used by the Krebs cycle enzyme succinate dehydrogenase), is always called: the metalloenzyme the metal-activated enzyme the holoenzyme the prosthetic group the cosubstratearrow_forwardDifferent enzymes that catalyse the same reaction are called O A. isoenzymes O B. holoenzymes O C. cofactors O D. coenzymes O E. agonists Which statement concerning the glycolytic and gluconeogenic pathways is correct? O A. Both are catabolic. O B. Both are anabolic. O C. Gluconeogenesis is catabolic while glycolysis is anabolic. O D. Gluconeogenesis is anabolic while glycolysis is catabolic. O E. Gluconeogenesis occurs in brain and glycolysis occurs in muscle. In lactic acid fermentation, which substance is oxidized and which is oxidized? O A. Lactate is reduced and NAD* is oxidized. OB. Lactate is reduced and pyruvate is oxidized. OC. Pyruvate is reduced and NADH is oxidized. O D. NADH is reduced and pyruvate is oxidized. O E. Lactate is oxidized and NADH is reduced.arrow_forward
- Please actually circle the substrates in the diagramarrow_forwardWhich one of the following is a "high-energy" biomolecule with a more negative AG° value for hydrolysis than ATP? 1,3-Bisphosphoglycerate Fructose-1,6-Bisphosphate Dihydroxyacetone Phosphate Glucose-6-Phosphate Glycerol-1-Phosphate O0000arrow_forwardMany enzyme mechanisms involve transferring protons. Sidechains of an enzyme can act as catalytic acids or catalytic bases in this process. The figure below shows a step in the reaction by the enzyme triose phosphate isomerase. Glu165 group. -OH Would a catalytic acid gain or lose a proton? Which sidechain in the mechanism is acting as a catalytic acid? co CH,OPO, 2- Activity remaining (%) Would a catalytic base gain or lose a proton? Which sidechain in the mechanism is acting as a catalytic base? 100 Curve I 8 Glu¹65 9 Below are two pH rate profiles showing the percent activity as a function of pH. Answer the questions below about how these curves relate to the mechanism of triose phosphate isomerase. Remember what fraction of a titratable group is unprotonated at the pka. 10 OH In this reaction a carbonyl is converted into an enol intermediate. Circle the enol 100 Activity remaining (%) H Curve 2 CIOH CIOH CH₂OPO,²- 8 9 N N 10 7 pH PH i) Which of these curves shows the pH rate…arrow_forward
- consider the following fatty acid: ch3-ch2-ch2-ch2-ch2-ch2=ch-cooh. in terms of reducing equivalents, how much energy will it yield upon complete oxidation of co2? - 4 molecules of NADH and 4 molecules of FADH2 -4 molecules of NADH and 3 moleculs of FADH2 - 19 molecules of NADH and 8 molecules of FADH2 - 5 molecules of NADH and 8 molecules of FADH2 - 19 molecules of NADH and 9 molecules of FADH2arrow_forwardWhich of these processes is most exergonic? (Think VERY carefully!) Dephosphorylation of glucose Conversion of enol to keto Oxidation of an aldehyde to a carboxylic acid group. ATP hydrolysisarrow_forwardLoss of this enzyme would be lethal to the cell CH₂-CH-CH₂ OH OH Glycerol- phosphate Loss of this enzyme would severely reduce the exchange of lipids between the cytosol and lumen P CH₂-CH-CH₂ O O I =C C=O Loss of this enzyme would lead to membrane phospholipids with identical phosphate head groups Phosphatase Acyl transferase Loss of this enzyme would result in membranes deficient in phosphatidylcholine Choline head Cytosol group ER lumen Choline phosphotransferase Loss of these enzymes would prevent lipid transfer between the ER and mitochondria Flippase lipid exchange proteinsarrow_forward
- All of the following compounds have sufficiently high group transfer potentials to lead to the production of ATP except: 1,3-bisphosphoglycerate. acetyl-CoA. O phosphocreatine. glucose-6-phosphate. phosphoenolpyruvate.arrow_forwardWhich class of enzymes have sigmoid or S-shaped plots of the rate of a reaction, v, versus the substrate concentration, [S], instead of the hyperbolic curves of the Michaelis-Menten equation? transferases holoenzymes O hydrolases O allosteric enzymes O ribozymesarrow_forwardlace the description with the appropriate step in the enzymatic reaction. The enzyme changes shape, resulting in an induced fit between substrate and enzyme. Glucose and galactose are released, and the enzyme is free to bind other substrates. Lactose Galactose Glucose- Substrate: Lactose Enzyme: Lactase 2 1 Enzyme-substrate complex The bond is broken between glucose and galactose 3 The substrate, lactose, binds to the enzyme forming an enzyme- substrate complexarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON