
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Need clarity about these kinds of questions. Thanks! :)
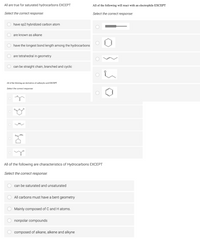
Transcribed Image Text:All are true for saturated hydrocarbons EXCEPT
All of the following will react with an electrophile EXCEPT
Select the correct response.:
Select the correct response:
have sp2 hybridized carbon atom
are known as alkane
have the longest bond length among the hydrocarbons
are tetrahedral in geometry
can be straight chain, branched and cyclic
All of the folowing are derivatives of carboxylic acid EXCEPT
Select the correct response:
O NH:
All of the following are characteristics of Hydrocarbons EXCEPT
Select the correct response:
can be saturated and unsaturated
All carbons must have a bent geometry
Mainly composed of C and H atoms.
nonpolar compounds
composed of alkane, alkene and alkyne
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- - 29 - g of water 3. Tequila often contains about 40 % alcohol by volume (80 proof) giving it a density of 0.812 g/mL. Calculate the mass, in grams, of a 5.0 mL shot of tequila. g of ice cm')arrow_forwardNeed help with homeworkarrow_forwardIn a suspension the substances are mixed at a: Ophysical level O atomic level O both physical and chemical level chemical levelarrow_forward
- A student measured the melting point range of an unknown solid to be 81.9 to 82.3 degrees celsius. a) Is the compound pure? b) Can you identify the compound from Table 2.2? c) If yes, what is the compound? Table 2.2: Solid - Melting Point (Celsius) Benzoic Acid - 122 Acentanilide - 114 Acetamide - 82 Biphenyl - 71 p-Dimethoxybenzene - 59 p-Dichlorobenzene - 54 Benzophenone - 48arrow_forward2) ENERGY TRANSFERS: Conduction, Convection, or Radiation Sunlight travels through space by the method. _Warm air moves around a room due to density differences. _You feel the warmth of a campfire. The spoon you are stirring your hot chocolate with gets hot on the end that you are holding. Ice melts while in your hand You burn your hand on the hot stove. The heater is located on the ground and the warm air rises. Does not require a MEDIUM to travel. a. b. C. d. e. f. 9. h. i.arrow_forwardChemistry HW need help, how to do these caculation and answer these questions?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY