
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
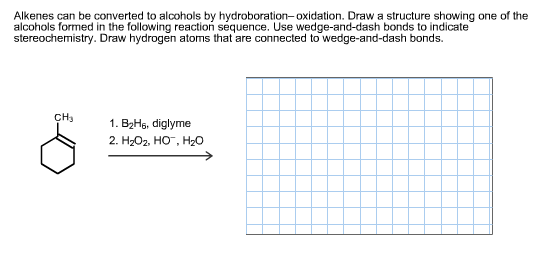
Transcribed Image Text:Alkenes can be converted to alcohols by hydroboration-oxidation. Draw a structure showing one of the
alcohols formed in the following reaction séquence. Use wedge-and-dash bonds to indicate
stereochemistry. Draw hydrogen atoms that are connected to wedge-and-dash bonds
CH3
1. B2Hg, diglyme
2. H-Ог, Но, H-о
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- What is the IUPAC name of the rearrangement product of 3,3-dimethyl-1-cyclopentene after the addition of HCl? 1,1-dimethyl-3-chlorocyclopentane 1,1-dimethyl-2-chlorocyclopentane 1,2-dimethyl-3-chlorocyclopentane 1,3-dimethyl-3-chlorocyclopentanearrow_forward2. Mention whether the given alcohols are positive for oxidation. Ethanol - Butanol (1-Butanol) - Isopropyl Alcohol (2-Propanol) - tert-Butyl Alcohol (2-Methyl-2-propanol) - 2-Methyl-2-butanol -arrow_forwardDraw a structural formula for 2-methylbutanoic acid. O • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. 8 CH4 #[ ] در ? ChemDoodlearrow_forward
- N,N-diethyl-m-toluamide (DEET) is the active ingredient in many insect repellent preparations. Following is one of the steps in its synthesis. In the box below draw the structure of the product of this reaction. H3C MgBr 1. CO₂ 2. H₂O* product • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading. • Draw the Grignard reagent as a covalent magnesium bromide.arrow_forward5. Write an equation showing the hydrobromination of the following alkene. Show major and minor products (if possible) and name the reactants and products using the IUPAC Nomenclature System.arrow_forwardDraw a structural formula for the substitution product of the reaction shown below. H3C CI CH3OH • Use the wedge/hash bond tools to indicate stereochemistry where it exists. • If more than one stereoisomer of product is formed, draw both. • Separate multiple products using the + sign from the drop-down menu.arrow_forward
- The table below shows the boiling point points of an alkane and aldehyde and an alcohol. Explain why the solubility of aldehydes and alcohols falls as the molecules get biggerarrow_forwardDraw a structural formula for the alkene you would use to prepare the alcohol shown by hydroboration/oxidation. OH AFFIL CH3 CH3 H • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. Sn [F ? ChemDoodlearrow_forwardWhat is the missing reactant R in this organic reaction? N H3O+ + R + H • Draw the structure of R in the drawing area below. Be sure to use wedge and dash bonds if it's necessary to draw one particular enantiomer. םarrow_forward
- Predict the major products of both organic reactions. Be sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. U + T но X Click and drag to start drawing a structure.arrow_forward10arrow_forwardDraw a structural formula for the alkene you would use to prepare the alcohol shown by hydroboration/oxidation CHCH3 OH •You do not have to consider stereochemistry. •You do not have to explicitly draw H atoms. • In cases where there is more than one answer, just draw one. 0▾ 4 n ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY