
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
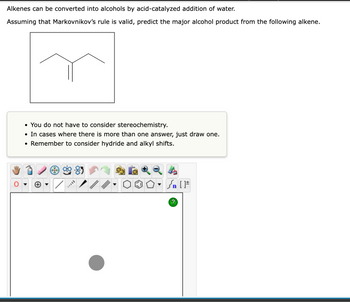
Transcribed Image Text:**Title: Alkene to Alcohol Conversion Using Markovnikov's Rule**
**Introduction:**
Alkenes can be converted into alcohols through an acid-catalyzed addition of water, following Markovnikov's rule. This rule assists in predicting the major alcohol product formed from alkenes.
**Problem Statement:**
Predict the major alcohol product from the following alkene, assuming that Markovnikov’s rule is valid.
**Alkene Structure:**
The image displays a structural diagram of an alkene with a branched chain, where a double bond is present between two of the carbon atoms in a straight chain of four carbon atoms.
**Guidelines:**
- **Stereochemistry Consideration:** Not required.
- **Multiple Answers:** If applicable, draw only one.
- **Hydride and Alkyl Shifts:** Consider these shifts in the mechanism.
**Diagram Explanation:**
The image shows a skeletal structure of a branched alkene. The double bond is located at the second carbon from one end of the chain. The structure is suitable for demonstrating an acid-catalyzed hydration reaction.
**Chemical Drawing Tools:**
The interface includes a toolbar with options for drawing various chemical structures and bonds, useful for illustrating predicted products.
**Conclusion:**
Using the guidelines and the structural diagram, apply Markovnikov's rule and consider possible shifts to determine the primary alcohol product obtained from the given alkene.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The reaction of the organoborane intermediate in the anti-Markovnikov hydration of an alkene is said to be an oxidation. The hydration of alkenes is said to be a reduction. What does this mean in terms of the total number of electrons of the alkene reactantarrow_forwardCharacterize EACH of the three given statements as being TRUE or FALSE and then indicate the collective true - false status of the statements using the choices. (1) Alcohols have higher boiling points than alkanes of similar molecular mass because of hydrogen bonding. (2) Polyols have similar boiling points to tertiary alcohols. (3) Primary and secondary alcohols give the same type of product when subjected to mild oxidizing agents. Group of answer choices All three statements are true. Two of the three statements are true. Only one of the statements is true. None of the statements are true.arrow_forwardMarkovnikov's rule for the addition of hydrogen halides to alkenes states that the incoming hydrogen bonds to the: sp² carbon with the most hydrogens already. sp carbon with the most hydrogens already. None of these choices is correct. sp² carbon with the fewest hydrogens already. sp³ carbon with the most hydrogens already.arrow_forward
- Isotretinoin is a retinoid derivative of vitamin A used in the treatment of severe recalcitrant acne. What is the degree of substitution of the encircled alkene functional group in its structure? HO, A trisubstituted monosubstituted tetrasubstituted D disubstitutedarrow_forwardWrite down the common (not IUPAC) names of the organic molecules that would be released if this molecule were hydrolyzed: CH,—O—C—(CH2)—CH=CH-CH,—CH=CH—(CH2) — CH3 CH-0- -(CH2)–CH=CH(CH2)CH3 O CH,—O-C=(CH2)=CH=CH(CH2)—CH3 Separate each name with a comma and a space. You will find useful information in the ALEKS Data resource. type your answer...arrow_forwardAlkenes can be converted to alcohols by reaction with mercuric acetate to form a β-hydroxyalkylmercury(II) acetate compound, a reaction called oxymercuration. Subsequent reduction with NaBH4 reduces the C–Hg bond to a C–H bond, forming the alkyl alcohol, a reaction called demercuration. Draw the structures of the Hg-containing compound(s) and the final alcohol product(s) formed in the following reaction sequence, omitting byproducts. If applicable, draw hydrogen at a chirality center and indicate stereochemistry via wedge-and-dash bonds.arrow_forward
- What alcohol is formed by the addition of water in the presence of sulfuric acid (an addition reaction) to 1-pentene?arrow_forwardName this aldehyde/ketone according to the IUPAC system. Include stereochemistry. CH3OCH₂CH2 H CH₂CH3arrow_forwardAlkenes can be converted to alcohols by reaction with mercuric acetate to form a β-hydroxyalkylmercury(II) acetate compound, a reaction called oxymercuration. Subsequent reduction with NaBH4 reduces the C–Hg bond to a C–H bond, forming the alkyl alcohol, a reaction called demercuration. Draw the structures of the Hg-containing compound(s) and the final alcohol product(s) formed in the following reaction sequence, omitting byproducts. If applicable, draw hydrogen at a chirality center and indicate stereochemistry via wedge-and-dash bonds.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY