
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
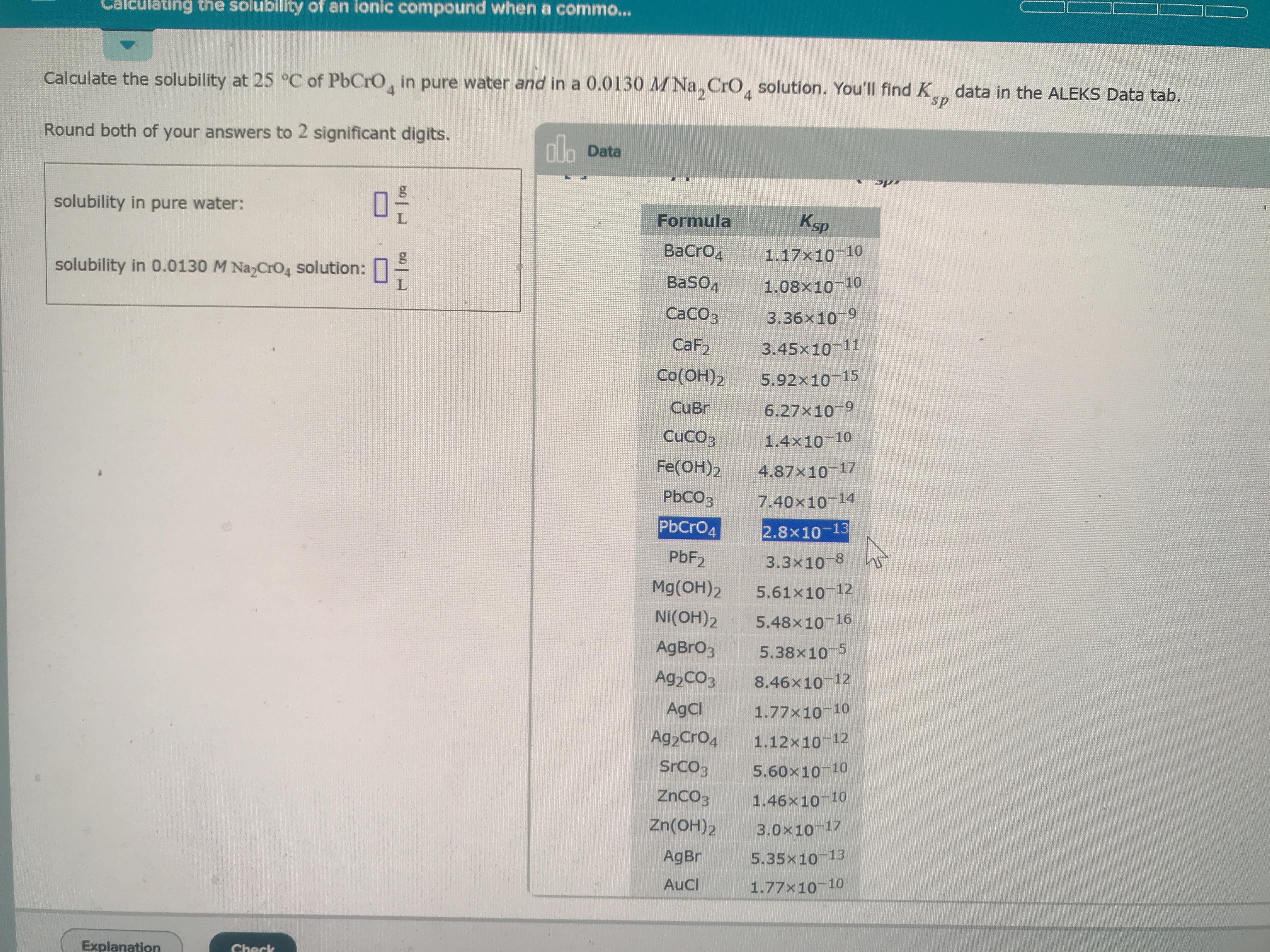
Calculated solely at 25 Celsius of PbCrO4 in pure water and in a .0130M. Na2CrO4 solution. Ksp of PbCrO4 is 2.8x10^-13
Transcribed Image Text:alculating the solubility of an ionic compound when a commo...
Calculate the solubility at 25 °C of PbCrO, in pure water and in a 0.0130 MNa, CrO, solution. You'll find K data in the ALEKS Data tab.
sp
Round both of your answers to 2 significant digits.
no Data
solubility in pure water:
Ksp
Formula
ВаCrОд
1.17x10 10
solubility in 0.0130 M Na CrO4 solution:
BaSO4
1.08x10 10
Сасоз
3.36x10 9
CaF2
3.45x10 11
Co(OH)2
5.92x10 15
CuBr
6.27x10-9
CuCO3
1.4x10 10
Fe(OH)2
4.87x10 17
PBCO3
7.40x10 14
PbCr04
2.8x10-13
PBF2
3.3x10-8
Mg(OH)2
5.61x10 12
NI(OH)2
5.48x10 16
AgBro3
5.38x10 5
Ag2CO3
8.46x10 12
AgCl
1.77x10 10
Ag2Cro4
1.12x10 12
Srco3
5.60x10 10
ZnCO3
1.46x10 10
Zn(OH)2
3.0x10 17
AgBr
5.35x10 13
AuCl
1.77x10 10
Explanation
Check
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 19" 0.00014 m Na2 Coz are combined? Determine the solubility of Cul (s) (Ksp.= 1.1x1013 In 0.15m Cu₂ (03 (aq) Solution,arrow_forwardCopper(I) ions in aqueous solution react with NH, (aq) according to Cut (aq) + 2 NH, (аq) — Си(NH, ); (aq) 3 6.3 х 1010 - Calculate the solubility (in g-L-!) of CuBr(s) (Ksp = 6.3 × 10-9) in 0.72 M NH, (aq). solubility of CuBr(s): g/Larrow_forwardIf a student combines specific amount CaCl2. 9H2O and Na2CO3 and obtained a solid precipitate. The student dissolve 1.37 gram of CaCl2. 9H2O in 25.0 mL of water and 0.984 g of Na2CO3 in 25.0 mL of water. The student is then combining the two solutions and observed the formation of a solid precipitate. The student filter the solution and recovered the precipitate. The following data were obtained for the experiment. Initial Mass of CaCl2. 9H2O 1.37 g Initial Mass of Na2CO3 0.984 g % yield of this experiment 87.4% 1. What is the balanced equation for this experiment?arrow_forward
- How would i estimate the number of micromoles of phosphate in tube 7, if the molarity of phosphate standard being 250.0 micromoles/liter?arrow_forwardTue Apr 11 TA ... B HO + 1. Write the chemical reaction for the titration of the filtered KHT solution in pure water.arrow_forward. A wastewater treatment plant discharges 10 MGD effluent containing 200 mg/L ultimate BOD into a stream flowing at 90 MGD with ultimate BOD of 5 mg/L. The deoxygenation constant, kd, is 0.12/day. (a) What is the ultimate BOD (BOD) remaining in the stream at a distance of 10,000 m downstream if the stream flows at a constant speed of 0.10 m/s? (b) What should be the treatment plant effluent BOD. (CW) in mg/L if the ultimate BOD, at 10,000 m downstream cannot exceed 5 mg/L?arrow_forward
- You added 0.05 mL of 0.10 M AgNOs to 4.0 ml of 2.0 M NaCI, and formed a saturated solution of AgCI (s):AgCI (s) 2Ag (ag)+ CI (aq)К = КэрUsing this saturated solution as the test solution you set up the following cellAg|Ag (test solution after reaction)llAg (1.0M)Agand measured Ecell = 0.61 V.(a) Using the measured value of Eceit and Eq (15), calculate the equilibrium [Ag ] (i.e. of the test solution after reaction).arrow_forwardTUOv to lls worla taum uo 1o9TIOoni bolam od lliw elimu iboo lut o 19 4. Given the Borax reaction below, what would be the solubility product (Ksp) of borax at 40 °C, if the solubility of borax is 0.8 mol L¯1 at 40 °C? Na,B4O5 (OH)4 · 8H,O(s) = 2Na+ (aq) + B405(OH) (aq) +8H,O(1) sr vol (A) gnsb vgotino brrbae ar bo CHO)gab xglerino bisbasie orb il Sarrow_forwardCan you please explain exactly how the denominator of (1-4S) is determined? Why it is 4S and not raised to the 4th?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY