
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Need help solve problem
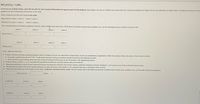
Transcribed Image Text:Al(C2H3O2)3 + CoBr2
In the first set of blanks below, enter the ion pairs for each reactant followed by the new ion pairs for the products. Each blank is for one jon. Within each blank enter the chemical symbol(s) and charge. Do not use subscripts or superscripts. If needed, see Activity 15 for
guidance on ionic compounds and Activity 16 for acids.
Enter a separate ion into each blank in this order:
REACTANTS: cation 1 anion 1 cation 2 anion 2
PRODUCTS: cation 1 anion 2
cation 2 anion 1
Text is provided above each blank as guidance, however, under extrente zoom (more than 150%) these text markers may become scrambled. If so, use the information above to obtain the proper order.
cation 1
anion 1
cation 2
anion 2
REACTANTS:
cation 1
anion 2
cation 2
anion 1
PRODUCTS:
Now, enter the following:
• Product chemical formulas (combined properly with no charges). Do not use subscripts or superscripts, but do use parentheses as appropriate. Make the products follow the order of the ion pairs entered.
O If you get a product ion pair of H'OH it will always become H200 as a product chemical formula in the balanced reaction.
O If the reaction is gas-evolving, enter the H20 as the 2nd product and the gas as the 3rd product, with appropriate phases.
• Product phases of either s, 4g or aq (within the provided parentheses). Reactant phases will not be entered.
Balancing coefficients in front of each chemical formula, including the reactants. Each whole number coefficient should be entered, including a 1. Do not leave any of the coefficient blanks empty.
The reactants are before the arrow and the products are after the arrow. Each product is on a separate line due to limitations within Canvas.
There are three blanks for each product in this order: coefficient, chemical formula, phase. The text markers above each blank may become scrambled under extreme zoom conditions, If so, use the order listed here as guidance.
AIC2H302)3 +
COB12
coefficient
product
phase
coefficient
product
phase
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A bucket of water has a volume of 0.473 kL . How many dL is this? STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 47300 1000 0.001 0.01 10 4730 0.0000473 100 0.473 0.1 1 0.00473 473 0.000473 kL dL +arrow_forwardINTRODUCTION Lab Data LABORATORY SIMULATION Mass of magnesium (g) Moles of magnesium (mol) Temperature of water (°C) Temperature of water (K) Vapor pressure of water (mmHg) Barometric pressure (mmHg) Observations Heated up and bubbled. Volume of hydrogen gas collected (mL) Volume of hydrogen gas collected (L) Pressure of hydrogen gas (mmHg) Pressure of hydrogen gas (atm) Moles of hydrogen gas produced (mol) Ideal gas constant (L-atm-mol-¹-K-¹) How to calculate ideal gas constant 0.014 0.00058 25.0 298.15 23.8 1.2 13.9 9 - X Water Vapor Pressure Table 14 15 Water Vapor Pressure Table Temperature (°C) 18.0 18.5 19.0 19.5 20.0 20.5 21.0 21.5 22.0 22.5 23.0 23.5 24.0 24.5 25.0 25.5 26.0 Pressure (mmHg) 15.5 16.0 16.5 17.0 17.5 18.1 18.6 19.2 19.8 20.4 21.1 21.7 22.4 23.1 23.8 24.5 25.2 Xarrow_forwardProperty Tested Observations Feels slippery Red Litmus Turns blue Phenolphthalein Turns pink Student 1 believes the data above represents an acid and student 2 thinks this is a base. Which student is correct? Explain your reasoning with at least 2 pieces of information.arrow_forward
- A solvent is added to a powdery pure solid in a beaker and stirred but not all of it dissolves. Which of the following terms could be used correctly to describe the remaining solid in the liquid? (Select all that apply). Suspended solid Insoluble impurity Crystals Precipitate Undissolved solid Washed solidarrow_forwardgenow.comiim/takeASSI [References) How many grams of sodium dichromate, Naz Cr207, should be added to a 250.0-mL volumetric flask to prepare 8.9 x 102 M Naz Crz 07 when the flask is filled to the mark with water? 6 item attempts remaining Submit Answer Try Another Version pt pt pt pt Previous Show Hint Email Instructor Cengage Learning | Cengage Technical Supportarrow_forwardAscorbic acid (C&H3O6) is also known as Vitamin C. What quantity in molecules of C&H:O6 does a Vitamin C drink with 1025 mg C&H:O6 contain? STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 5.68 x 10-3 2.925 x 10-16 1025 2.925 x 1022 0.010 88.07 6.022 x 1022 3.505 x 10-2 2.925 x 1016 3.505x 1024 1 6.022 x 1023 2.925 x 10-19 176.12 0.001 100 1000 2.925 x 10-22 5.68 x 1020 3.505 x 1021 mol C&H&O6 mg C6H&O6 g/mol C&H&O6 molecules CsH&O6 g C6H&Oarrow_forward
- Was the gradual color change observed when the sodium thiosulfate (Na2S2O3) crystal was added to the aqueous solution of KI/I2 in Station C evidence of a chemical or physical change? Group of answer choices chemical change physical changearrow_forwardExercise 3 is already answeredarrow_forwardDilution Practice How many mL of HCI, 0.1 M, will you need to prepare 0.700 L of HCL, 0.025 M? (Ans. 175 mL of aqueous HCI, 0.1 M} Write the problem set up that justifies the answer. 1 DELL 女 23 $ & 3 6. 7 t i W e r y u d f g h jarrow_forward
- Which of the following is true of electrostatic forces but not gravitational forces? (Select all that apply.) Are mediated by fields Require the presence of two or more objects Can be attractive or repulsive Increase as the mass of the objects increase Increase as the charge of the objects increase Increase as the distance between the objects increases Save Answerarrow_forwardChemistry pakoarrow_forward22:11 Question 21 of 30 Submit Determine the amount in grams of KCI that exists in 20.3 g of a solution that contains 1.14 % KCl by mass STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) 23.1 0.272 g KCI 6.022 x 1023 17.8 mol KCI 74.55 20.3 %m KCI 1.14 0.014 g solution 100 g КC/mol 1 1450 0.231 Tap here or pull up for additional resourcesarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY