
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
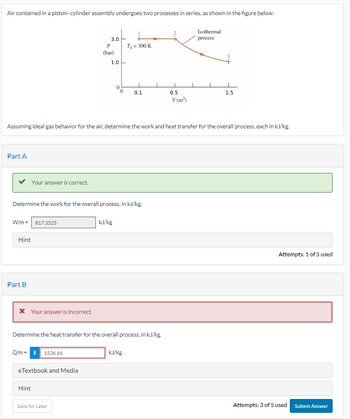
Transcribed Image Text:Air contained in a piston-cylinder assembly undergoes two processes in series, as shown in the figure below.
Part A
Your answer is correct.
W/m = 817.3525
Hint
Part B
* Your answer is incorrect.
Determine the work for the overall process, in kJ/kg.
Assuming ideal gas behavior for the air, determine the work and heat transfer for the overall process, each in kJ/kg.
Q/m=11536.66
3.0
eTextbook and Media
P
(bar)
Hint
1.0
Save for Later
Determine the heat transfer for the overall process, in kJ/kg.
T₁ = 300 K
kJ/kg
0.1
kJ/kg
0.5
V (m³)
Isothermal
process
1.5
Attempts: 1 of 5 used
Attempts: 3 of 5 used
Submit Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A piston-cylinder assembly contains a mystery substance that undergoes a series of processes. Process 1-->2: Constant pressure process at 5bar from v1=.07m3/kg to v2=.12m3/kg Process 2->3: Constant volume process to saturated vapor Process 3->4: Constant temperature process to quality of 50% Process 4->5: Constant volume process to 5bar Sketch the processes on Pt, Pv, and Tv, plots. Lable the axes including property values; use closed dots to show the states, use solid lines to connect the states, add number and arrows to make clear the states numbers and process directions. Could this be considered a thermodynamic cycle? Why or why not?arrow_forward2arrow_forwardCalculate the work done in kJ from state 1 to state 4, 1W4, for the process shown in the P-v diagram below. The system contains 2 kg of air. P [kPa] 300- 4 200 3 1 100 - 0.2 0.4 0.6 v [m/kg] 3 2.arrow_forward
- just part d answer options are 1.8kJ 1.3J 1.3kJ 1.8Jarrow_forwardA piston/cylinder apparatus initially contains 0.4 kg of saturated water vapor at a pressure of 200 kPa. The water vapor is now heated until the (extensive) volume of the H,0 is 0.5 m³ (state 2). (a) Fill in the following table. H,0 State Temp. T(C) P (kPa) Specific volume Volume Specific internal energy u (kJ/kg) 2529.1 Pressure P, = 200 kPa V (m?) Sat. vapor v (m³/kg) 1 200 0.5 state 1. (b) Determine the total work done by the H20 during the process, 1→2. (c) Determine the total heat transfer to the H,0 during the process, 1→2. (d) Draw the process on a P-v diagram. Label both states. (e) EXTRA CREDIT: Determine the change in enthalpy of the H20 from 1>2 and explain why this answer makes sense.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY