
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
please help with the product/solution of the picture:
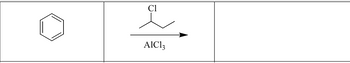
Transcribed Image Text:AIC13
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 1 You are employed as a co-op student at the Drug and Alcohol Testing Association of Canada (DATAC) developing analytical tests for sports doping agents. You are asked to prepare a procedure for the extraction of methylphenidate, the active compound in Ritalin, from urine samples (consider them as simple aqueous layers, you do not need to consider other components!). The goal of the procedure is to extract the methylphenidate into an organic layer which will then be evaporated and the residue will be tested for the drug. You find that methylphenidate is highly soluble in 2-methyltetrahydrofuran, a bio-renewable solvent. Draw the structure of 2-methyltetrahydrofuran and give two reasons why it is a good solvent choice for liquid-liquid extraction. (Please find the image attached below to help you with this question) Question 2 (cont. from scenario 1). Your colleague is helping you develop the urine test. They suggest that the urine should be adjusted to a pH above 7 before…arrow_forwardWhat type of reaction is shown below? PbSO4(aq) + 2HCl(aq) ==> PbCl2(s) + H2SO4(aq) What is produced?arrow_forwardDraw all the structures of crystal violet as pH is increased from 0-14 (I need the drawing to be done using the computer, not a handwritten drawing please).arrow_forward
- How would you prepare 9 liters of 50% dye solution beginning with 60%sye solution?arrow_forwardPlease don't provide handwriting solutionarrow_forward9. Assume the exact mass for compound X is 151.9472. a. What is the molecular formula, b. What is the index of hydrogen deficiency. 73 100 90 80- 70- 60- 50 40에 152 30- 20- 107 135 55 10- 26 18 0- 10 150 160 170 20 30 40 50 60 70 80 90 100 110 120 130 140 30- 25- 20- 15- 10어 3500 3000 2500 2000 1500 1000 Wavenumber (cm-1) c. Draw 2 structures that are consistent with the data -812 914 -1137 1265 --1342 -1395 1432 1717 -2571 -2670 3067 Transmittancearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY